Vitamin A Supplementation in Infants
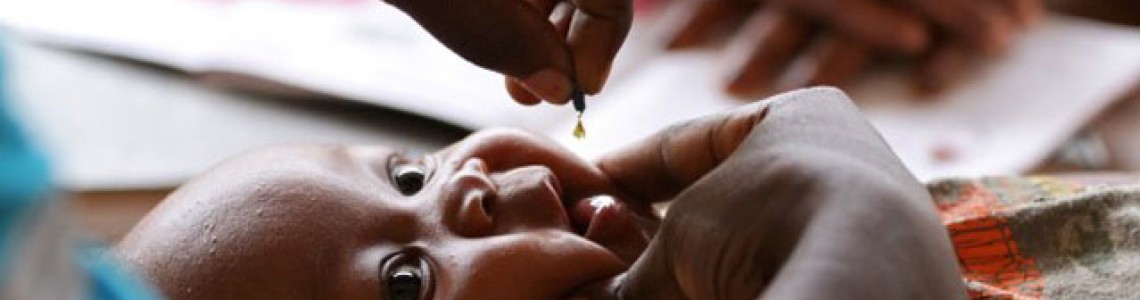
Vitamin A supplementation in infants and children 6–59 months of age
WHO recommendations Guidance summary*
In settings where vitamin A deficiency is a public health problem** (prevalence of night blindness is 1% or higher in children 24–59 months of age or where the prevalence of vitamin A deficiency (serum retinol 0.70 µmol/l or lower) is 20% or higher in infants and children 6–59 months of age), high-dose vitamin A supplementation is recommended in infants and children 6–59 months of age.
Suggested vitamin A supplementation scheme for infants children 6–59 months of age:
Target group — Infants 6–11 months of age (including HIV+) — Children 12–59 months of age (including HIV+)
Dose — 100 000 IU (30 mg RE) vitamin A — 200 000 IU (60 mg RE) vitamin A
Frequency — Once — Every 4-6 months
Route of administration — Oral liquid, oil-based preparation of retinyl palmitate or retinyl acetate
Settings — Populations where the prevalence of night blindness is 1% or higher in children 24–59 months of age or where the prevalence of vitamin A deficiency (serum retinol 0.70 μmol/l or lower) is 20% or higher in infants and children 6–59 months of age.
IU, international units; RE, retinol equivalent. a. An oil-based vitamin A solution can be delivered using soft gelatin capsules, as a single-dose dispenser or a graduated spoon (2). The consensus among manufacturers to use consistent color coding for the different doses in soft gelatin capsules, namely red for the 200 000 IU capsules and blue for the 100 000 IU capsules, has led to much-improved training and operational efficiencies in the field.
Remarks
- This guideline replaces previous recommendations on vitamin A supplementation for the prevention of vitamin A deficiency, xerophthalmia, and nutritional blindness in infants and children 6–59 months of age (3).
- The above recommendation can also be applied in populations where infants and children may be infected with HIV.
- The magnitude of the effect may differ across settings and populations, possibly due to the extent of vitamin A deficiency or the availability of other nutrients (e.g. dietary intake of vitamin A will differ across locations and the effects of supplementation may be smaller in places with greater access to vitamin A-rich foods or with regular consumption of vitamin A-fortified foods).
- This intervention should be used along with other strategies to improve vitamin A intakes, such as dietary diversification (4) and food fortification (5).
- Adverse effects within 48 hours of receiving supplements containing 100 000–200 000 IU vitamin A are usually mild and transient, with no longterm consequences. Adverse effects may include bulging of open fontanelles in younger infants, and nausea and/or vomiting and headache in older children with closed fontanelles.
- Vitamin A supplements should be delivered to children 6–59 months of age twice yearly, during health system contacts. This should be marked on the child health card, or integrated into other public health programmes aimed at improving child survival, such as polio or measles national immunization days, or biannual child health days delivering a package of interventions such as deworming, distribution of insecticide-treated mosquito nets and immunizations.
- A quality assurance process should be established to guarantee that supplements are manufactured, packaged and stored in a controlled and uncontaminated environment (6).
- When determining the vitamin A status of a population, guidelines on indicators for assessing vitamin A deficiency should be referred to (7, 8).
- Recommendations for the treatment of xerophthalmia and the use of vitamin A supplements during episodes of measles are not covered in this guideline. Existing guidelines on the treatment of xerophthalmia and measles in infants and children 6–59 months of age should be referred to in these cases (3, 9).
* This is an extract from the relevant guideline (10). Additional guidance information can be found in this document.
** Determination of vitamin A deficiency as a public health problem involves estimating the prevalence of deficiency in a population by using specific biochemical and clinical indicators of vitamin A status. Classification of countries based on the most recent estimates is available in the guidance document, Global prevalence of vitamin A deficiency in populations at risk 1995–2005 (1).
Case Study – UNICEF INDIA
Vitamin A supplementation: a national good news story
Record coverage attained, with 62 million under-fives protected from vitamin A deficiency in just one year
The World Health Organization (WHO) recommends that in vitamin A-deficient areas, children six months to five years should receive a preventive dose of vitamin A supplementation every six months. While India’s vitamin A program follows this recommendation, a 2006 National Family Health Survey indicated that only 25 percent of under-fives were receiving supplementation. Further analysis showed children missed by the program would benefit greatly, as they were more likely to be undernourished and belong to vulnerable families. The study also showed states with higher under-five mortality rates had lower vitamin A supplementation coverage.
Recognizing the problem, the government that same year adopted biannual supplementation to reach out to children under-five with the following regime:
- Children below one year receive the first vitamin A supplementation dose with their routine measles immunization at nine months.
- For children aged one to five years, the subsequent nine doses of vitamin A supplementation be administered twice a year, six months apart, through a biannual large-scale outreach vitamin A supplementation strategy.
Currently, 15 of India’s major states are taking part in this biannual outreach strategy in partnership with UNICEF and others. UNICEF’s role has been to support state governments’ capacities to source and distribute vitamin A supplements to districts and blocks on time while mobilizing families and communities to bring their children to take advantage of the scheme.
As a result of the program, the proportion of children receiving two doses of vitamin A annually – referred to as “full vitamin A supplementation coverage” – increased from a quarter in 2006 to two-thirds in 2011, with seven of India’s 15 major states reporting full coverage rates of more than 80 percent. In 2011 alone, a record 62 million children were protected. Importantly, between 2007 and 2011.
Refer below documents for more information:

2522 Comment(s)
Kinguin bietet fantastische Aktionen und Deals auf eine breite Auswahl an Spielen und Software!
Perfect!
People often think their old cars are worthless, but there’s still value in them. Scrap car removal services buy vehicles regardless of their condition. Whether it's rusty, damaged, or doesn’t run, they’ll offer you cash and haul it away for free. It’s a great way to free up space and make some money without the hassle.
https://aeonmining.com/
https://www.morecashforscrap.com/locations/coquitlam?srsltid=https://vancouverseoagency.ca/
https://aeonmining.com/xml/index.html
https://365bitcoinminer.com/
https://365bitcoinminer.com/xml/index.html
https://bchminer.com/xml/index.html
https://bchminer.com
https://www.epsxf.com
https://www.yyeps.com
https://epsxf.com
https://yyeps.com
https://eps-machine.net
https://epp-machine.com
https://eptu-machine.com
https://eps-machine.top
https://epp-eptu-machine.com
https://sw-eps.com
https://www.sw-eps.com
https://www.epp-eptu-machine.com
https://www.eps-machine.top
https://www.eptu-machine.com
https://www.epp-machine.com
https://www.eps-machine.net
https://aeonmining.com/
https://aeonmining.com/xml/index.html
https://365bitcoinminer.com/
https://365bitcoinminer.com/xml/index.html
https://bchminer.com/xml/index.html
https://bchminer.com
https://epp-machine.com/ca3-ETPU-Machine
https://sw-eps.com/gongsi/74.html
https://eps-machine.net/ETPU-Machine
https://eptu-machine.com/ETPU-Machine
https://eptu-machine.com/ETPU-Machine-news
https://eptu-machine.com/EPS-Machine
https://eptu-machine.com/EPP-Machine
https://eptu-machine.com/EPS-Mould
https://epp-machine.com/ca3-ETPU-Machine
https://eps-machine.net/EPS-Machine
https://eps-machine.net/EPP-Machine
https://eps-machine.net/ETPU-Machine
https://eps-machine.net/EPS-Mould
https://sw-eps.com/EPS-Machine
https://sw-eps.com/ETPU-Machine
https://sw-eps.com/EPP-Machine
https://epsxf.com/EPS-Machine/
https://epsxf.com/EPS-Mould/
https://epsxf.com/ETPU-Machine/
https://epsxf.com/EPP-Machine/
https://epsxf.com/EPS-Machine-news/
https://epp-machine.com/ca1-EPS-Machine/
https://epp-machine.com/ca2-EPP-Machine/
https://jingcheng-seo.com
http://jingcheng-seo.com/seo-news/
http://jingcheng-seo.com/case-seo/
http://jingcheng-seo.com/service/
http://jingcheng-seo.com/contact/
http://jingcheng-seo.com/about/
http://free-web-tool.com/
http://free-web-tool.com/bot/
http://free-web-tool.com/COMO/
http://free-web-tool.com/estrategia/
http://free-web-tool.com/Fortune-Tiger/
http://free-web-tool.com/Como-Jogar-Fortune-Tiger/
http://www.free-web-tool.com/
http://www.free-web-tool.com/bot/
http://www.free-web-tool.com/COMO/
http://www.free-web-tool.com/estrategia/
http://www.free-web-tool.com/Fortune-Tiger/
http://www.free-web-tool.com/Como-Jogar-Fortune-Tiger/
https://epp-eptu-machine.com/sitemap.xml
https://jingcheng-seo.com/sitemap.xml
https://www.epp-eptu-machine.com/sitemap.xml
https://www.jingcheng-seo.com/sitemap.xml
https://www.eps-machine.top/sitemap.xml
https://eps-machine.top/sitemap.xml
https://free358.com/sitemap.xml
https://www.free358.com/sitemap.xml
https://free-web-tool.com/sitemap.xml
https://www.free-web-tool.com/sitemap.xml
https://9999tiger.com/sitemap.xml
https://13slots.com/sitemap.xml
https://ru.sw-eps.com/
https://ru.sw-eps.com/EPS-Machine/
https://ru.sw-eps.com/EPP-Machine/
https://ru.sw-eps.com/ETPU-Machine/
https://ru.sw-eps.com/EPS-Machine-news/
https://ru.sw-eps.com/ETPU-machine-new/
https://ru.sw-eps.com/sitemap.xml
https://03topgame.com/
https://gamesimes.com/
https://gamesimes.com/id/
https://gamesimes.com/vie/
https://gamesimes.com/in/
https://gamesimes.com/may/
https://gamesimes.com/ru/
https://gamesimes.com/de/
https://gamesimes.com/per/
https://gamesimes.com/ar/
https://gamesimes.com/jp/
https://gamesimes.com/en/
https://gamesimes.com/sitemap.xml
https://gamesimes.com/id/sitemap.xml
https://gamesimes.com/vie/sitemap.xml
https://gamesimes.com/in/sitemap.xml
https://gamesimes.com/may/sitemap.xml
https://gamesimes.com/ru/sitemap.xml
https://gamesimes.com/de/sitemap.xml
https://gamesimes.com/per/sitemap.xml
https://gamesimes.com/ar/sitemap.xml
https://gamesimes.com/jp/sitemap.xml
https://gamesimes.com/en/sitemap.xml
Effective time management is a crucial skill for students to acquire, and assignment writing offers an ideal platform for its development. Assignments come with deadlines, prompting students to prioritize tasks, allocate time, and work efficiently. By practicing time management, students learn to balance their academic responsibilities, extracurricular activities, and personal commitments. These skills extend beyond the realm of assignments, benefiting students throughout their academic journey and professional careers.
Get ready to sparkle this holiday season with the Edison Jacket Christmas Sale! This year, we’re making your festive shopping even more exciting with an exclusive 50% OFF on our iconic jackets. Whether you’re looking for the perfect gift or a show-stopping piece for your holiday wardrobe, our collection has something for everyone. From timeless leather classics to bold sequin statement pieces, each jacket is crafted with care to combine style, comfort, and quality. Don’t miss this opportunity to celebrate the season in style and save big. Shop now and make this Christmas truly unforgettable with Edison Jacket!
https://www.oxminer.com/xml/index.html#/
https://www.oxminer.com/
https://www.oxminer.com/xml
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/website
https://winseoer.com/roobts
https://winseoer.com/roobts
https://winseoer.com/roobts
https://winseoer.com/roobts
https://winseoer.com/roobts
https://winseoer.com/roobts
https://winseoer.com/roobts
https://winseoer.com/seo123
https://winseoer.com/seo123
https://winseoer.com/seo123
https://winseoer.com/seo123
https://winseoer.com/seo123
https://winseoer.com/seo123
https://winseoer.com/seoliuhen
https://winseoer.com/seoliuhen
https://winseoer.com/seoliuhen
https://winseoer.com/seoliuhen
https://winseoer.com/seoliuhen
https://winseoer.com/seoliuhen
https://winseoer.com/Google-link
https://winseoer.com/Google-link
https://winseoer.com/Google-link
https://winseoer.com/Google-link
https://winseoer.com/Google-link
https://winseoer.com/Google-PR
https://winseoer.com/Google-PR
https://winseoer.com/Google-PR
https://winseoer.com/Google-PR
https://winseoer.com/Google-PR
https://winseoer.com/google-seo
https://winseoer.com/google-seo
https://winseoer.com
https://winseoer.com
https://winseoer.com
https://winseoer.com
https://winseoer.com/GooglePlay
https://winseoer.com/GooglePlay
https://winseoer.com/GooglePlay
https://winseoer.com/GooglePlay
https://winseoer.com/GooglePlay
https://winseoer.com/game-seo
https://winseoer.com/game-seo
https://winseoer.com/game-seo
https://winseoer.com/game-seo
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
I genuinely appreciate your effort in creating this post and sharing your perspective. It has helped broaden my knowledge and provided me with a new way of looking at things. Please continue to share such valuable content, as it makes a positive difference in the lives of your readers. I look forward to reading more of your posts in the future, as I am certain they will continue to provide me with useful information and fresh perspectives. Keep up the great work!
https://winseoer.com
https://www.eps-machine.net/
https://ara.eps-machine.net/
https://t.me/s/googlebaping
https://t.me/s/googleboot2025
https://t.me/s/googlekuaipai
https://t.me/s/googlelinkgo
https://t.me/s/googleseoliuhen
https://t.me/s/CityCallGirls
https://t.me/s/NationalGirlfriend
https://t.me/s/CallGirlService2025
https://i76lfbrsbs3y.com
https://bibw9q9k.com
https://rushexfqbv.com
https://fpvktnldkc.com
https://gjrifyismh.com
https://jgmjewcmbb.com
https://onjefxsaij.com
https://gdfwtkunbu.com
https://enbmepumqy.com
https://zxxtubdrbf.com
https://pbapzqsycl.com
https://beibmexcpg.com
https://fncnxdirmx.com
https://gigcinnluj.com
https://ipqoowwohu.com
https://rtsoxpxqss.com
https://hwzcaacimn.com
https://edwmuaemqe.com
https://xjeozpqlsm.com
https://zcnyiukjfk.com
https://skuwjckyee.com
https://luxytjdrif.com
https://hkxxwbxkaa.com
https://ez-corners.com
https://obagkop.com
https://chaygia.com
https://st303.com
https://sxjiepeng.com
https://saunaspy.com
https://csm-uscc.com
https://e-fangzhi.com
https://rugbychef.com
https://oooku2014.com
https://zeytin-tr.com
https://mafiasos.com
https://buy-coolz.com
https://dzaishang.com
https://stepinz.com
https://tongtian168.com
https://cctuoxun.com
https://detki38.com
https://alix-edc.com
https://zenduder.com
https://nbcaijia.com
https://luppopants.com
https://batergyn.com
https://hrbjinzhong.com
https://mingsheng-v.com
https://jiequansy.com
https://mutebag.com
https://vse-nomera.com
https://pengjunzhubao.com
https://dfd555.com
https://ccowomen.com
https://jiaoxuejiqiao.com
https://plutojobs.com
https://187dc.com
https://adpoket.com
https://kac980.com
https://uec913.com
https://iks686.com
https://pey402.com
https://afh845.com
https://sga901.com
https://vln416.com
https://mvu470.com
https://fvw234.com
https://ivd131.com
https://uyl837.com
https://kpl809.com
https://leg107.com
https://pzs139.com
https://poe686.com
https://saz390.com
https://guf502.com
https://lvp259.com
https://svg518.com
https://lgl394.com
https://emi479.com
https://tai454.com
https://gaw296.com
https://les275.com
https://enm302.com
https://ucy593.com
https://weu796.com
https://kuh160.com
https://owv926.com
https://ncz248.com
https://ipo814.com
https://ukm485.com
https://iws189.com
https://fdp247.com
https://ziv939.com
https://wnm604.com
https://mok670.com
https://naf750.com
https://ucy590.com
https://kgl710.com
https://hoe572.com
https://cku909.com
https://gvd549.com
https://dht14.com
https://uhs72.com
https://szc95.com
https://rya13.com
https://jwd43.com
https://rpn98.com
https://yrh69.com
https://vte78.com
https://evn34.com
https://uhv606.com
https://fgn421.com
https://hze435.com
https://rcp667.com
https://nvn641.com
https://okt339.com
https://gmw673.com
https://ymk953.com
https://ipu152.com
https://niy819.com
https://lcg907.com
https://pui471.com
https://pzy265.com
https://gln453.com
https://wdv892.com
https://lso673.com
https://kgy41.com
https://uya49.com
https://jpw81.com
https://urt83.com
https://jyc19.com
https://3388xg.com
https://qdfenjin.com
https://groove-to-go.com
https://tourmerapi.com
https://lfweixiu.com
https://nmhuayuan.com
https://daiken-k.com
https://hongquanlx.com
https://cssanwei.com
https://yinxing788.com
https://rtbltda.com
https://e-siho.com
https://least1.com
https://kma-ok.com
https://kingcue.com
https://zzshenghong.com
https://zhuzhoupb.com
https://yueyongsuliao.com
https://ncyinguang.com
https://boxbc.com
https://2glue.com
https://bmwmen.com
https://biush.com
https://xinbeil.com
https://gnomlens.com
https://de1pro.com
https://zajii.com
https://cp934.com
https://sreditor.com
https://bolilaw.com
https://qfnue.com
https://mic352.com
https://wlu108.com
https://whi344.com
https://xej585.com
https://mmy351.com
https://peoplewhat.com
https://ni234.com
https://ecyzyf.com
https://forme-sante.com
https://frigimar.com
https://hnxxsh.com
https://izengood.com
https://gdpy1.com
https://emprehost.com
https://lotobars.com
https://teoti-b.com
https://ardelacalle.com
https://scarxtape.com
https://cibospizza.com
https://nikholas.com
https://dimaconsa.com
https://ruedelis.com
https://bjd-sheyes.com
https://datsumo-m.com
https://safetspin.com
https://ayoungco.com
https://rapsva.com
https://cattich.com
https://verardfarms.com
https://dunav75.com
https://eyetalkies.com
https://muaall.com
https://manbeertech.com
https://reklama11.com
https://stolinovi.com
https://xwg18.com
https://sberrybrandy.com
https://okullargida.com
https://cellulicontrol.com
https://rockfallsfiber.com
https://doctorscruises.com
https://ecosystempaper.com
https://y2anetwork.com
https://garconfacile.com
https://ersoylarkaroseri.com
https://papermaculture.com
https://medicalivraison.com
https://thekcsource.com
https://frognirvanagifts.com
https://malmraz.com
https://mygotostore.com
https://desmondrealtor.com
https://callscarlet.com
https://colomfagundo.com
https://vbvmarketing.com
https://vantagecards.com
https://ufbowling.com
https://ponponforever.com
https://insportstv.com
https://wpproficom.com
https://avarmour.com
https://soloadx.com
https://rustilift.com
https://collicit.com
https://catertrac.com
https://ebslatino.com
https://enteksolar.com
https://kezhiquan.com
https://okfraud.com
https://painintel.com
https://trashore.com
https://amykiss.com
https://806back.com
https://unicornvn.com
https://domsoum.com
https://crepespot.com
https://haltgain.com
https://6bbitech.com
https://payguruji.com
https://oriyasong.com
https://dizpari.com
https://misgina.com
https://av.run/
https://av.run/
https://av.run/
https://av.run/index.php/vod/type/id/15.html
https://av.run/index.php/vod/type/id/1.html
https://av.run/index.php/vod/type/id/2.html
https://av.run/index.php/vod/type/id/4.html
https://av.run/index.php/vod/type/id/5.html
https://av.run/index.php/vod/type/id/6.html
https://av.run/index.php/vod/type/id/19.html
https://av.run/index.php/vod/type/id/11.html
https://av.run/index.php/vod/type/id/12.html
<a href=https://easypsychedelic.com/product/buy-1plsd/ rel="dofollow">Buy 1PLSD</a>
<a href=https://easypsychedelic.com/product/1plsd-for-sale/ rel="dofollow">1plsd for sale</a>
<a href=https://easypsychedelic.com/product/buying-lsd-online/ rel="dofollow">Buying LSD Online</a>
<a href=https://easypsychedelic.com/product/buy-oxycodone-online/ rel="dofollow">Buy Oxycodone Online</a>
<a href=https://easypsychedelic.com/product/peyote-for-sale/ rel="dofollow">Peyote For Sale</a>
<a href=https://easypsychedelic.com/product/order-magic-mushrooms-online/ rel="dofollow">Order Magic Mushrooms Online</a>
<a href=https://easypsychedelic.com/product/buy-magic-mushrooms-online/ rel="dofollow">Buy Magic Mushrooms Online</a>
<a href=https://easypsychedelic.com/product/5-meo-dmt-for-sale/rel="dofollow">5 MEO DMT For Sale</a>
<a href=https://easypsychedelic.com/product/dmt-for-sale/rel="dofollow">DMT For Sale</a>
<a href=https://easypsychedelic.com/product/buy-dmt-online/rel="dofollow">Buy DMT Online</a>
<a href=https://easypsychedelic.com/product/dabs-for-sale/rel="dofollow">Dabs For Sale</a>
<a href=https://easypsychedelic.com/product/buy-hash-online/rel="dofollow">Buy Hash Online</a>
<a href=https://easypsychedelic.com/product/moroccan-hash/rel="dofollow">Moroccan Hash</a>
<a href=https://easypsychedelic.com/product/hash-for-sale/rel="dofollow">Hash For Sale</a>
<a href=https://easypsychedelic.com/product/lebanese-blonde-hash/ rel="dofollow">Lebanese Blonde Hash</a>
<a href=https://easypsychedelic.com/product/buy-shrooms-online/rel="dofollow">Buy Shrooms Online</a>
<a href=https://easypsychedelic.com/product/tidal-wave-mushroom/rel="dofollow">Tilda Wave Mushroom</a>
<a href=https://easypsychedelic.com/product/lsd-for-sale/rel="dofollow">LSD For Sale</a>
<a href=https://easypsychedelic.com/product/k2-sheets-for-sale/rel="dofollow">K2 Sheet For Sale</a>
<a href=https://easypsychedelic.com/product/k2-spice-spray-diablo-amazon/rel="dofollow">K2 Spice Spray Diablo Amazon</a>
<a href=https://easypsychedelic.com/product/buy-k2-spice/rel="dofollow">Buy K2 Spice</a>
<a href=https://easypsychedelic.com/product/mephedrone-for-sale/rel="dofollow">Mephedrone For Sale</a>
<a href=https://easypsychedelic.com/product/buy-mephedrone-online/rel="dofollow">Buy Mephedrone Online</a>
<a href=https://easypsychedelic.com/product/buy-ketamine/rel="dofollow">Buy Ketamine</a>
<a href=https://easypsychedelic.com/product/ketamine-for-sale/rel="dofollow">Ketamine For Sale</a>
<a href=https://easypsychedelic.com/product/buy-codeine-online/rel="dofollow">Buy Codeine Online</a>
<a href=https://easypsychedelic.com/product/2cb-for-sale/rel="dofollow">2cb For Sale</a>
<a href=https://easypsychedelic.com/product/buy-tramadol-online/rel="dofollow">Buy Tramadol Online</a>
<a href=https://easypsychedelic.com/product/buy-xanax-online-overnight/ rel="dofollow">Buy Xanax Online Overnight</a>
<a href=https://easypsychedelic.com/product/buy-valium-online/rel="dofollow">Buy Valium Online</a>
<a href=https://easypsychedelic.com/product/buy-ativan-online/rel="dofollow">Buy Ativan Online</a>
<a href=https://easypsychedelic.com/product/buy-ghb-online/rel="dofollow">Buy GHB Online</a>
<a href=https://easypsychedelic.com/product/buy-fentanyl-online/rel="dofollow">Buy Fentanyl Online</a>
<a href=https://easypsychedelic.com/product/buy-hydrocodone-online/rel="dofollow">Buy Hydrocodone Online</a>
<a href=https://easypsychedelic.com/product/buy-dilaudid-online/rel="dofollow">Buy Dilaudid Online</a>
<a href=https://easypsychedelic.com/product/buy-methadone-online/rel="dofollow">Buy Methadone Online</a>
<a href=https://easypsychedelic.com/product/buy-crystal-meth-online/rel="dofollow">Buy Crystal Meth</a>
<a href=https://easypsychedelic.com/product/buy-1plsd/ rel="dofollow">Buy 1PLSD</a>
<a href=https://easypsychedelic.com/product/1plsd-for-sale/ rel="dofollow">1plsd for sale</a>
<a href=https://easypsychedelic.com/product/buying-lsd-online/ rel="dofollow">Buying LSD Online</a>
<a href=https://easypsychedelic.com/product/buy-oxycodone-online/ rel="dofollow">Buy Oxycodone Online</a>
<a href=https://easypsychedelic.com/product/peyote-for-sale/ rel="dofollow">Peyote For Sale</a>
<a href=https://easypsychedelic.com/product/order-magic-mushrooms-online/ rel="dofollow">Order Magic Mushrooms Online</a>
<a href=https://easypsychedelic.com/product/buy-magic-mushrooms-online/ rel="dofollow">Buy Magic Mushrooms Online</a>
<a href=https://easypsychedelic.com/product/5-meo-dmt-for-sale/rel="dofollow">5 MEO DMT For Sale</a>
<a href=https://easypsychedelic.com/product/dmt-for-sale/rel="dofollow">DMT For Sale</a>
<a href=https://easypsychedelic.com/product/buy-dmt-online/rel="dofollow">Buy DMT Online</a>
<a href=https://easypsychedelic.com/product/dabs-for-sale/rel="dofollow">Dabs For Sale</a>
<a href=https://easypsychedelic.com/product/buy-hash-online/rel="dofollow">Buy Hash Online</a>
<a href=https://easypsychedelic.com/product/moroccan-hash/rel="dofollow">Moroccan Hash</a>
<a href=https://easypsychedelic.com/product/hash-for-sale/rel="dofollow">Hash For Sale</a>
<a href=https://easypsychedelic.com/product/lebanese-blonde-hash/ rel="dofollow">Lebanese Blonde Hash</a>
<a href=https://easypsychedelic.com/product/buy-shrooms-online/rel="dofollow">Buy Shrooms Online</a>
<a href=https://easypsychedelic.com/product/tidal-wave-mushroom/rel="dofollow">Tilda Wave Mushroom</a>
<a href=https://easypsychedelic.com/product/lsd-for-sale/rel="dofollow">LSD For Sale</a>
<a href=https://easypsychedelic.com/product/k2-sheets-for-sale/rel="dofollow">K2 Sheet For Sale</a>
<a href=https://easypsychedelic.com/product/k2-spice-spray-diablo-amazon/rel="dofollow">K2 Spice Spray Diablo Amazon</a>
<a href=https://easypsychedelic.com/product/buy-k2-spice/rel="dofollow">Buy K2 Spice</a>
<a href=https://easypsychedelic.com/product/mephedrone-for-sale/rel="dofollow">Mephedrone For Sale</a>
<a href=https://easypsychedelic.com/product/buy-mephedrone-online/rel="dofollow">Buy Mephedrone Online</a>
<a href=https://easypsychedelic.com/product/buy-ketamine/rel="dofollow">Buy Ketamine</a>
<a href=https://easypsychedelic.com/product/ketamine-for-sale/rel="dofollow">Ketamine For Sale</a>
<a href=https://easypsychedelic.com/product/buy-codeine-online/rel="dofollow">Buy Codeine Online</a>
<a href=https://easypsychedelic.com/product/2cb-for-sale/rel="dofollow">2cb For Sale</a>
<a href=https://easypsychedelic.com/product/buy-tramadol-online/rel="dofollow">Buy Tramadol Online</a>
<a href=https://easypsychedelic.com/product/buy-xanax-online-overnight/ rel="dofollow">Buy Xanax Online Overnight</a>
<a href=https://easypsychedelic.com/product/buy-valium-online/rel="dofollow">Buy Valium Online</a>
<a href=https://easypsychedelic.com/product/buy-ativan-online/rel="dofollow">Buy Ativan Online</a>
<a href=https://easypsychedelic.com/product/buy-ghb-online/rel="dofollow">Buy GHB Online</a>
<a href=https://easypsychedelic.com/product/buy-fentanyl-online/rel="dofollow">Buy Fentanyl Online</a>
<a href=https://easypsychedelic.com/product/buy-hydrocodone-online/rel="dofollow">Buy Hydrocodone Online</a>
<a href=https://easypsychedelic.com/product/buy-dilaudid-online/rel="dofollow">Buy Dilaudid Online</a>
<a href=https://easypsychedelic.com/product/buy-methadone-online/rel="dofollow">Buy Methadone Online</a>
<a href=https://easypsychedelic.com/product/buy-crystal-meth-online/rel="dofollow">Buy Crystal Meth</a>
I love the authenticity in your blog posts! Every read feels like a conversation with a friend, and the ideas shared always inspire me to think deeper and explore more
I love the authenticity in your blog posts! Every read feels like a conversation with a friend, and the ideas shared always inspire me to think deeper and explore more
http://www.drmlminer.com/xml/favicon.ico?t=1
https://sixmining.com
https://www.sixmining.com
https://sixmining.com/xml/favicon.ico
https://cesurminings.com/xml/favicon.ico
https://paladinmining.com/
https://paladinmining.com/xml/favicon.ico
https://advancedminers.com
https://advancedminers.com/xml/favicon.ico
https://richminer.com
https://taozseo.com/
https://blockchaincloudmining.com/home.html
https://blockchaincloudmining.com/login.html
https://blockchaincloudmining.com/register.html
https://blockchaincloudmining.com/about.html
https://blockchaincloudmining.com/app.html
https://blockchaincloudmining.com/blog.html
https:/blockchaincloudmining.com
https:/blockchainpe.com
https:/blockchainspecs.com
https:/blockchaincloudmining.com/xml/favicon.ico
https://xyminers.com/
https://xyminers.cc
https://xyminers.vip
https://xyminers.com/xml/index.html
https://xyminers.com/xml/favicon.ico
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://xyminers.cc/home.html
https://xyminers.cc/product.html
https://xyminers.cc/register.html
https://xyminers.cc/login.html
https://xyminers.cc/about.html
https://xyminers.cc/app.html
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://megatechammunution.com/
https://megatechammunution.com/product/trail-boss-powder/
https://megatechammunution.com/product/blackhorn-209
https://megatechammunution.com/product/vortex-crossfire-reddot/
https://megatechammunution.com/product/leupold-deltapoint-pro/
https://megatechammunution.com/product/eotech-magnifier/
https://megatechammunution.com/product/labradar/
https://megatechammunution.com/product/apex-trigger/
https://megatechammunution.com/product/swagger-bipods/
https://megatechammunution.com/product/timney-trigger/
https://megatechammunution.com/product/para-15-trigger/
https://megatechammunution.com/product/alamo-15-trigger/
https://megatechammunution.com/product/glock-performance-trigger/
https://megatechammunution.com/product/geissele-trigger/
https://megatechammunution.com/product/cmc-triggers/
https://megatechammunution.com/product/binary-trigger/
https://megatechammunution.com/product/wot-trigger/
https://megatechammunution.com/product/holosun-507k/
https://reptileexotica.com/product/camel-spider/
https://reptileexotica.com/product/zero-bearded-dragon-for-sale/
https://reptileexotica.com/product/red-eyed-crocodile-skink-for-sale/
https://reptileexotica.com/product/banana-ball-python/
https://reptileexotica.com/product/blue-tongue-skink-for-sale/
https://reptileexotica.com/product/armadillo-lizard-for-sale/
https://reptileexotica.com/product/leachie-gecko/
https://reptileexotica.com/product/white-lipped-python-for-sale/
https://reptileexotica.com/product/giant-african-millipede-for-sale/
https://reptileexotica.com/product/frilled-dragons-for-sale/
https://reptileexotica.com/product/emerald-tree-boa-for-sale/
https://reptileexotica.com/product/schneider-skink/
https://reptileexotica.com/product/bold-leopard-gecko-for-sale/
https://reptileexotica.com/product/dumerils-boa-for-sale/
https://reptileexotica.com/product/blue-eyed-leucistic-ball-python-for-sale/
https://reptileexotica.com/product/blood-python-for-sale/
https://reptileexotica.com/product/black-rat-snake-for-sale/
https://reptileexotica.com/product/black-boa/
https://reptileexotica.com/product/baby-razorback-musk-turtles-baby-turtles/
https://reptileexotica.com/product/baby-hermanns-tortoise/
https://reptileexotica.com/product/veiled-chameleon-for-sale/
https://reptileexotica.com/product/tarantulas-for-sale/
https://reptileexotica.com/product/spider-ball-python-for-sale/
https://reptileexotica.com/product/red-bearded-dragon/
https://reptileexotica.com/product/panda-pied-ball-python/
https://reptileexotica.com/product/leucistic-leopard-gecko/
https://reptileexotica.com/product/leopard-tortoise-for-sale/
https://reptileexotica.com/product/egyptian-uromastyx/
https://reptileexotica.com/product/western-hognose-snake-for-sale/
https://alphaspirituality.com
https://alphaspirituality.com/product/purple-mdma/
https://alphaspirituality.com/product/oxynorm/
https://alphaspirituality.com/product/rybelsus-weight-loss/
https://alphaspirituality.com/product/buy-ozempic-online/
https://alphaspirituality.com/product/buy-wegovy-online/
https://alphaspirituality.com/product/percocet-30s/
https://alphaspirituality.com/product/adderall-uk
https://alphaspirituality.com/product/ketamine-for-anxiety/
https://alphaspirituality.com/product/pregabalin-bnf/
https://alphaspirituality.com/product/mifegest-buy-online/
https://alphaspirituality.com/product/lions-mane-mushrooms-near/
https://alphaspirituality.com/product/lions-mane-uk/
https://alphaspirituality.com/product/buy-mifepristone/
https://alphaspirituality.com/product/dihydrocodeine-30mg/
https://alphaspirituality.com/product/cytotec-uk
https://alphaspirituality.com/product/mdma-buy/
https://alphaspirituality.com/product/blue-tesla-pills/
https://alphaspirituality.com/product/mdma-buying/
https://alphaspirituality.com/product/mdma-for-depression-treatment/
https://razevapeofficial.com/product/raz-vape-flavors/
https://razevapeofficial.com/product/raz-vapes/
https://razevapeofficial.com/product/raz-disposable-vape/
https://razevapeofficial.com/product/miami-mint/
https://razevapeofficial.com/product/raz-ruby-flavor/
https://razevapeofficial.com/product/raz-flavors/
https://razevapeofficial.com/product/raz-vicky-flavor/
https://razevapeofficial.com/product/tiffany-raz-flavor/
https://razevapeofficial.com/product/watermelon-ice-vape/
https://razevapeofficial.com/product/raz-night-crawler/
https://razevapeofficial.com/product/razz-vape/
<a href=https://dejevudreambarsofficial.com/product/lyt-chocolate-bar-thc// rel="dofollow">lyt chocolate bar thc</a>
<a href=https://dejevudreambarsofficial.com/product/psilo-vibin-chocolate-bar// rel="dofollow">psilo vibin chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/trippy-flip-chocolate-bar// rel="dofollow">trippy flip chocolate bar</a>
<a hrer=https://dejevudreambarsofficial.com/product/tesla-bar-chocolate// rel="dofollow">tesla bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/wonder-bar-chocolate// rel="dofollow">wonder bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/azure-chocolate// rel="dofollow">azure chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/dejavu-dream-chocolate-bar// rel="dofollow">dejavu dream chocolate bar</a>
<a href=https://dejevudreambarsofficial.com/product/magic-mushroom-candy-bar// rel="dofollow">magic mushroom candy bar</a>
<a href=https://dejevudreambarsofficial.com/product/trehouse-gummies// rel="dofollow">trehouse gummies</a>
<a href=https://dejevudreambarsofficial.com/product/polka-dot-mushroom-chocolate// rel="dofollow">polka dot mushroom chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/space-bar-chocolate// rel="dofollow">space bar chocolate</a>
<a href=https://dejevudreambarsofficial.com/product/happy-bars-920// rel="dofollow">happy bars 920</a>
<a href=https://dejevudreambarsofficial.com/product/mantra-chocolate-bars// rel="dofollow">mantra chocolate bars</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Bar Mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu dream mushroom</a>
<a href="https://dejavudreambars.com/" rel="dofollow">Deja Vu Dream Mushroom Chocolate Bar</a>
<a href="https://dejavudreambars.com/product/neau-tropics-artisanal-chocolate-mazapan-horchata/" rel="dofollow">neautropics</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candies</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom candy</a>
<a href="https://dejavudreambars.com/product/mibblers-mushroom-sweet-tarts/" rel="dofollow">Mibblers mushroom</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast edibles</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast gummies</a>
<a href="https://dejavudreambars.com/product/baja-blast-edibles/" rel="dofollow">baja blast</a>
<a href="https://dejavudreambars.com/" rel="dofollow">deja vu</a>
<a href="https://dejavudreambars.com/product/tesla-bar-chocolate/" rel="dofollow">tesla bar chocolate</a>
<a href="https://dejavudreambars.com/product/wonderbar-mushroom-chocolate/" rel="dofollow">wonderbar mushroom chocolate</a>
<a href="https://dejavudreambars.com/product/wonder-bar-mushrooms/" rel="dofollow">wonder bar mushrooms</a>
<a href="https://dejavudreambars.com/product/wonder-bar-shroom/" rel="dofollow">wonder bar shrooms</a>
<a href=https://alphaautomobilecarparts.com//rel="dofollow">370z nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/oil-catch-can/rel="dofollow">oil catch can</a>
<a href=https://alphaautomobilecarparts.com/product/resonator-delete/rel="dofollow">resonator delete</a>
<a href=https://alphaautomobilecarparts.com/product/nismo-wheels/rel="dofollow">nismo wheels</a>
<a href=https://alphaautomobilecarparts.com/product/corsa-exhaust/rel="dofollow">corsa exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/honda-steering-wheel/rel="dofollow">honda steering wheel</a>
<a href=https://alphaautomobilecarparts.com/product/te37-wheels/rel="dofollow">te37 wheels</a>
<a href=https://alphaautomobilecarparts.com/product/bbs-rims/rel="dofollow">bbs rims</a>
<a href=https://alphaautomobilecarparts.com/product/blitz-03/rel="dofollow">blitz 03</a>
<a href=https://alphaautomobilecarparts.com/product/x-pipe-exhaust/rel="dofollow">x pipe exhaust</a>
<a href=https://alphaautomobilecarparts.com/product/ford-raptor-exhaust-tip/rel="dofollow">ford raptor exhaust tip</a>
<a href=https://alphaautomobilecarparts.com/product/borla-exhaust/rel="dofollow">borla exhaust</a>
<a href=https://alphaautomobilecarparts.com/product-category/370z-nismo-rims//rel="dofollow">370z nismo rims</a>
<a href=https://alphaautomobilecarparts.com/product/transmission-filter/rel="dofollow">transmission filter</a>
<a href=https://pistachiochocolatebars.com/product/feastables-chocolate/" rel="dofollow">feastables chocolate</a>
<a href=https://pistachiochocolatebars.com/product/mr-beast-chocolate/" rel="dofollow">mr beast chocolate</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-near-me/" rel="dofollow">pistachio chocolate bar near me</a>
<a href=https://pistachiochocolatebars.com/product/pistachio-chocolate-bar-dubai/" rel="dofollow">pistachio chocolate bar dubai</a>
<a href=https://pistachiochocolatebars.com/product/dubai-pistachio-chocolate-bar/" rel="dofollow">dubai pistachio chocolate bar</a>
https://earnmining.com/home.html
https://earnmining.com/product.html
https://earnmining.com/register.html
https://earnmining.com/login.html
https://earnmining.com/about.html
https://earnmining.com/app.html
https://winnermining.com/home.html
https://winnermining.com/login.html
https://winnermining.com/register.html
https://winnermining.com/product.html
https://winnermining.com/about.html
https://winnermining.com/app.html
https://winnermining.com/blog.html
https://savvymining.com/home.html
https://savvymining.com/product.html
https://savvymining.com/FAQ.html
https://savvymining.com/login.html
https://savvymining.com/register.html
https://savvymining.com/affiliate.html
https://savvymining.com/blog.html
https://savvymining.com/about.html
https://savvymining.com/welfare.html
https://savvymining.com/tutorial.html
https://savvymining.com/app.html
https://savvymining.com/privacy.html
https://savvymining.com/terms.html
https://savvymining.com/contact.html
https://savvymining.com/Bounty.html
https://xyminers.com/home.html
https://xyminers.com/product.html
https://xyminers.com/register.html
https://xyminers.com/login.html
https://xyminers.com/about.html
https://xyminers.com/app.html
https://savvymining.net/home.html
https://savvymining.net/product.html
https://savvymining.net/FAQ.html
https://savvymining.net/login.html
https://savvymining.net/register.html
https://savvymining.net/affiliate.html
https://savvymining.net/blog.html
https://savvymining.net/about.html
https://savvymining.net/welfare.html
https://savvymining.net/tutorial.html
https://savvymining.net/app.html
https://savvymining.net/privacy.html
https://savvymining.net/terms.html
https://savvymining.net/contact.html
https://savvymining.net/Bounty.html
https://savvymining.cc/home.html
https://savvymining.cc/product.html
https://savvymining.cc/FAQ.html
https://savvymining.cc/login.html
https://savvymining.cc/register.html
https://savvymining.cc/affiliate.html
https://savvymining.cc/blog.html
https://savvymining.cc/about.html
https://savvymining.cc/welfare.html
https://savvymining.cc/tutorial.html
https://savvymining.cc/app.html
https://savvymining.cc/privacy.html
https://savvymining.cc/terms.html
https://savvymining.cc/contact.html
https://savvymining.cc/Bounty.html
https://xyminers.cc/home.html
https://xyminers.cc/product.html
https://xyminers.cc/register.html
https://xyminers.cc/login.html
https://xyminers.cc/about.html
https://xyminers.cc/app.html
https://earnmining.com/home.html
https://earnmining.com/product.html
https://earnmining.com/register.html
https://earnmining.com/login.html
https://earnmining.com/about.html
https://earnmining.com/app.html
https://winnermining.com/home.html
https://winnermining.com/login.html
https://winnermining.com/register.html
https://winnermining.com/product.html
https://winnermining.com/about.html
https://winnermining.com/app.html
https://winnermining.com/blog.html
https://savvymining.com/home.html
https://savvymining.com/product.html
https://savvymining.com/FAQ.html
https://savvymining.com/login.html
https://savvymining.com/register.html
https://savvymining.com/affiliate.html
https://savvymining.com/blog.html
https://savvymining.com/about.html
https://savvymining.com/welfare.html
https://savvymining.com/tutorial.html
https://savvymining.com/app.html
https://savvymining.com/privacy.html
https://savvymining.com/terms.html
https://savvymining.com/contact.html
https://savvymining.com/Bounty.html
https://xyminers.com/home.html
https://xyminers.com/product.html
https://xyminers.com/register.html
https://xyminers.com/login.html
https://xyminers.com/about.html
https://xyminers.com/app.html
https://savvymining.net/home.html
https://savvymining.net/product.html
https://savvymining.net/FAQ.html
https://savvymining.net/login.html
https://savvymining.net/register.html
https://savvymining.net/affiliate.html
https://savvymining.net/blog.html
https://savvymining.net/about.html
https://savvymining.net/welfare.html
https://savvymining.net/tutorial.html
https://savvymining.net/app.html
https://savvymining.net/privacy.html
https://savvymining.net/terms.html
https://savvymining.net/contact.html
https://savvymining.net/Bounty.html
https://savvymining.cc/home.html
https://savvymining.cc/product.html
https://savvymining.cc/FAQ.html
https://savvymining.cc/login.html
https://savvymining.cc/register.html
https://savvymining.cc/affiliate.html
https://savvymining.cc/blog.html
https://savvymining.cc/about.html
https://savvymining.cc/welfare.html
https://savvymining.cc/tutorial.html
https://savvymining.cc/app.html
https://savvymining.cc/privacy.html
https://savvymining.cc/terms.html
https://savvymining.cc/contact.html
https://savvymining.cc/Bounty.html
https://xyminers.cc/home.html
https://xyminers.cc/product.html
https://xyminers.cc/register.html
https://xyminers.cc/login.html
https://xyminers.cc/about.html
https://xyminers.cc/app.html
https://hrminer.com/home.html
https://hrminer.com/login.html
https://hrminer.com/register.html
https://hrminer.com/about.html
https://hrminer.com/app.html
https://hrminer.com/blog.html
https://hrminer.com/
https://bitcoinist.com/btc-reaches-a-new-high-why-has-hr-miner-become-the-first-choice-for-investors-in-mining/
https://szamoldki.hu/hu/hirek/a-felhoalapu-banyaszat-trendjei-2025-ben-hogyan-lehet-biztonsagosan-es-hatekonyan-kriptoeszkozokhoz-jutni
https://videolista.hu/videok/video/a-felhoalapu-banyaszat-trendjei-2025-ben-hogyan-lehet-biztonsagosan-es-hatekonyan-kriptoeszkozokhoz-jutni-9907
https://kriptotarca.hu/a-felhoalapu-banyaszat-trendjei-2025-ben-hogyan-lehet-biztonsagosan-es-hatekonyan-kriptoeszkozokhoz-jutni/
https://bitcoinworld.co.in/crypto-global-latest-news-savvy-mining-teaches-you-how-to-make-more-than-10000-yuan-a-day-in-cloud-mining/
https://en.cryptonomist.ch/2025/03/31/maple-ceo-hopes-make-savvy-mining-new-pillar-global-cloud-mining/
https://primeirahora.com.br/o-ceo-da-maple-espera-tornar-a-savvy-mining-um-novo-pilar-da-mineracao-em-nuvem-global-permitindo-que-cada-usuario-ganhe-facilmente-us-100-000/
https://cyprus-mail.com/2025/04/01/contact-savvy-mining-dont-just-read-the-news-we-teach-you-to-become-the-person-in-the-news
https://blockzeit.com/contact-savvy-mining-dont-just-read-the-news-we-teach-you-to-become-the-person-in-the-news/
https://bitcoinist.com/maple-ceo-hopes-to-make-savvy-mining-a-new-pillar-of-global-cloud-mining-allowing-each-user-to-easily-earn-100000/
https://bitcoinist.com/btc-is-highly-volatile-and-the-golden-age-of-choosing-savvy-mining-has-arrived-with-daily-venue-exceeding-10000-yuan/
https://blockonomi.com/maple-ceo-hopes-to-make-savvy-mining-a-new-pillar-of-global-cloud-mining-allowing-each-user-to-easily-earn-100000/
https://www.thecryptoupdates.com/maple-ceo-hopes-to-make-savvy-mining-a-new-pillar-of-global-cloud-mining-allowing-each-user-to-easily-earn-100000/
https://finbold.com/savvy-mining-strongly-recommends-antminers21-xp-hyd-allowing-every-user-to-earn-passive-venue/ (https://finbold.com/savvy-mining-strongly-recommends-antminers21-xp-hyd-allowing-every-user-to-earn-passive-income/)
https://moderndiplomacy.eu/2025/04/01/doge-price-prediction-2025-how-to-get-doge-at-low-cost-through-savvy-mining-structed/
https://www.newsbtc.com/press-releases/savvy-mining-provides-legal-secure-and-stable-cloud-computing-services-that-promise-every-user-to-be-profitable/
https://finbold.com/bitcoin-holdings-data-expose-individual-holdings-still-dominate-etfs-companies-and-governments-account-for-only-10-earning-10000-a-day-is-easy/
https://walletinvestor.com/magazine/do-you-want-to-achieve-financial-freedom-savvy-mining-the-most-popular-project-in-april-makes-5000-a-day
https://captainaltcoin.com/btc-is-highly-volatile-the-golden-age-of-choosing-savvy-mining-has-arrived-with-daily-venue-exceeding-10000-yuan/
https://bitcoinist.com/report-states-that-xrp-has-brought-profits-to-88-of-savvy-mining-cloud-mining-investors/
https://coinedition.com/how-to-make-money-online-savvy-mining-teaches-you-how-to-make-500-20000-a-day/
https://blockchainreporter.net/maple-ceo-hopes-to-make-savvy-mining-a-new-pillar-of-global-cloud-mining-allowing-each-user-to-easily-earn-100000/
https://coinfomania.com/smart-cryptocurrency-investors-made-200000-in-profit-using-savvy-mining-in-march/
https://hellomagyar.hu/2025/04/02/a-btc-rendkivul-valtozekony-elerkezett-a-savvy-mining-valasztasanak-aranykora/
https://dailynewshungary.com/contact-savvy-mining-dont-just-read-the-news-we-teach-you-to-become-the-person-in-the-news/
https://coincentral.com/doge-price-prediction-2025-how-to-get-doge-at-low-cost-through-savvy-mining-structed/
https://techbullion.com/doge-price-prediction-2025-how-to-get-doge-at-low-cost-through-savvy-mining-structed/
https://www.analyticsinsight.net/cryptocurrency-analytics-insight/btc-is-highly-volatile-and-the-golden-age-of-choosing-savvy-mining-has-arrived-with-daily-venue-exceeding-10000-yuan
https://www.analyticsinsight.net/cryptocurrency-analytics-insight/dont-worry-if-xrp-is-sideways-lets-make-the-coin-move-savvy-mining-helps-you-earn-10000-a-day
https://crypto.news/innovation-and-combination-xrp-enthusiasts-teach-users-how-to-earn-with-savvy-mining/
https://tradebrains.in/brand/dogecoin-will-be-a-new-trend-in-2025-choose-savvy-mining-and-enjoy-the-growth-of-wealth/
https://www.cryptopolitan.com/maple-ceo-hopes-to-make-savvy-mining-a-new-pillar-of-global-cloud-mining-allowing-each-user-to-easily-earn-100000/
https://mascolombia.com/en/best-cloud-mining-platform-in-2025-savvy-mining/
https://www.thehansindia.com/business/trump-vigorously-promotes-cryptocurrency-savvy-cloud-mining-promotes-global-financial-innovation-958025
https://bitcoinist.com/xrp-doge-enthusiasts-earn-8541-daily-with-savvy-mining-cloud-mining/
https://dgmnews.com/posts/as-trumps-tariff-concerns-caused-bitcoin-and-xrp-to-fall-many-investors-flocked-to-savvr-mining-and-made-an-easy-profit-of-36670-a-day/
https://samsungmagazine.eu/2025/04/07/as-trumps-tariff-concerns-caused-bitcoin-and-xrp-to-fall-many-investors-flocked-to-savvr-mining-and-made-an-easy-profit-of-36670-a-day/
https://praguemorning.cz/tariff-policy-triggers-cryptocurrency-fluctuations-investors-turn-to-savvr-mining-to-earn-a-steady-15880-daily/
https://programminginsider.com/the-most-stable-investment-in-2025-choose-btc-cloud-mining-and-earn-10000-passive-venue-every-day-through-savvy-mining/
https://mignews.com/news/lifestyle/torgovye-poshliny-vyzvali-kolebaniya-kriptorynka.html
https://thetradable.com/opinions/low-threshold-and-high-return-savvy-mining-takes-you-to-easily-earn-btc-every-day
https://profitline.hu/mikozben-trump-vamintezkedesei-miatt-a-bitcoin-es-az-xrp-arfolyama-visszaesett-szamos-befekteto-a-savvy-mining-fele-fordult-es-napi-36-670-dollaros-egyszeru-nyereseget-ert-el-472942
https://cryptwerk.com/post/use-six-mining-cloud-mining-service-to-easily-earn-7500-per-day-accessible-worldwide/
https://blockchainreporter.net/use-six-mining-cloud-mining-service-to-easily-earn-7500-per-day-accessible-worldwide/
https://www.analyticsinsight.net/cryptocurrency-analytics-insight/use-six-mining-cloud-mining-service-to-easily-earn-7500-per-day-accessible-worldwide
https://tynmagazine.com/bitcoin-continua-aumentando-hasta-los-us-9-400-y-los-poseedores-de-bitcoin-acuden-en-masa-a-six-mining-para-minar-obteniendo-ganancias-diarias-de-us-10-000/
https://www.newsbtc.com/press-releases/bitcoin-is-about-to-experience-a-second-surge-six-mining-guides-users-to-earn-0-3-bitcoins-per-day-online/
https://usawire.com/cryptocurrency-market-value-rises-six-mining-online-guidance-earns-5000-a-days/
https://bitcoinist.com/it-only-takes-3-minutes-to-mine-btc-with-six-mining/
https://coinfomania.com/earn-passive-income-with-cryptocurrency-through-six-minings-cloud-mining-platform/
https://www.periodistadigital.com/economia/bolsa/20250428/six-mining-ensena-ganar-5-200-dolares-dia-internet-noticia-689405083962/
https://www.bulnews.bg/article/485876
Seeking a reliable SEO company in Stockholm for SEO optimization, online marketing, website design, Google Ads management, Social Media, USA, UK, Australia, UAE.
We provide support for those looking for 'Take my GED for me' or 'Take my TEAS exam' solutions. Need help with your GED or TEAS exam? We offer services so you can pay someone to take your GED or TEAS exam, hire someone for exam assistance and solutions
https://cryptominingfirm.com/
https://cryptominingfirm.com/home.html
https://cryptominingfirm.com/login.html
https://cryptominingfirm.com/register.
https://cryptominingfirm.com/about.html
https://cryptominingfirm.com/app.html
https://cryptominingfirm.com/blog.html
https://aptminer.com/home.html
https://aptminer.com/login.html
https://aptminer.com/register.html
https://aptminer.com/about.html
https://aptminer.com/blog.html
https://aptminer.com/app.html
https://aptminer.com/
https://xrpmining.com/
https://juminer.com/
https://juminer.com/page/about.html
https://juminer.com/page/faq.html
https://juminer.com/page/tutorial.html
https://all4mining.com/
https://sunnymining.com/
https://sunnymining.com/xml/favicon.ico
https://www.sunnymining.com/
https://www.sunnymining.com/xml/favicon.ico
https://www.sunnymining.com/home.html
https://www.sunnymining.com/login.html
https://www.sunnymining.com/register.html
https://www.sunnymining.com/about.html
https://www.sunnymining.com/app.html
https://www.sunnymining.com/blog.html
https://www.sunnymining.com/
https://www.sunnymining.com/xml/favicon.ico
https://www.sunnymining.com/home.html
https://www.sunnymining.com/login.html
https://www.sunnymining.com/register.html
https://www.sunnymining.com/about.html
https://www.sunnymining.com/app.html
https://www.sunnymining.com/blog.html
https://bayminer.com/
https://bayminer.com/home.html
https://bayminer.com/product.html
https://bayminer.com/register.html
https://bayminer.com//about.html
https://bayminer.com/app.html
https://bayminer.com/FAQ.html
https://bayminer.com/affiliate.html
You seem to be very knowledgable about this topic.
https://quidminer.com
https://quidminer.com/home.html
https://quidminer.com/login.html
https://quidminer.com/register.html
https://quidminer.com/affiliate.html
https://quidminer.com/about.html
https://quidminer.com/blog.html
https://quidminer.com/app.html
https://bayminer.com/
https://bayminer.com/home.html
https://bayminer.com/product.html
https://bayminer.com/register.html
https://bayminer.com//about.html
https://bayminer.com/app.html
https://bayminer.com/FAQ.html
https://bayminer.com/affiliate.html
https://www.bayminer.com/
https://www.bayminer.com/home.html
https://www.bayminer.com/product.html
https://www.bayminer.com/register.html
https://www.bayminer.com//about.html
https://www.bayminer.com/app.html
https://www.bayminer.com/FAQ.html
https://www.bayminer.com/affiliate.html
https://bjmining.com/home.html
https://bjmining.com/login.html
https://bjmining.com/register.html
https://bjmining.com/app.html
https://bjmining.com/about.html
https://bjmining.com/affiliate.html
https://bjmining.com/
https://bjmining.com/xml/favicon.ico
https://www.bjmining.com/xml/favicon.ico
https://www.bjmining.com/
https://www.bjmining.com/home.html
https://www.bjmining.com/login.html
https://www.bjmining.com/register.html
https://www.bjmining.com/app.html
https://www.bjmining.com/about.html
https://www.bjmining.com/affiliate.html
https://nr7miner.com/
https://www.rimining.com
https://www.rimining.com/home.html
https://www.rimining.com/login.html
https://www.rimining.com/register.html
https://www.rimining.com/app.html
https://www.rimining.com/blog.html
https://www.rimining.com/about.html
https://www.rimining.com/favicon.ico
https://www.sunnymining.com/
https://www.sunnymining.com/xml/favicon.ico
https://www.sunnymining.com/home.html
https://www.sunnymining.com/login.html
https://www.sunnymining.com/register.html
https://www.sunnymining.com/about.html
https://www.sunnymining.com/app.html
https://www.sunnymining.com/blog.html
https://nr7miner.com/
https://optominer.com/favicon.ico
https://optominer.com
https://optominer.com/home.html
https://optominer.com/login.html
https://optominer.com/register.html
https://optominer.com/app.html
https://optominer.com/blog.html
https://optominer.com/about.html
https://www.optominer.com/favicon.ico
https://www.optominer.com
https://www.optominer.com/home.html
https://www.optominer.com/login.html
https://www.optominer.com/register.html
https://www.optominer.com/app.html
https://www.optominer.com/blog.html
https://www.optominer.com/about.html
https://bayminer.com/
https://bayminer.com/home.html
https://bayminer.com/product.html
https://bayminer.com/register.html
https://bayminer.com//about.html
https://bayminer.com/app.html
https://bayminer.com/FAQ.html
https://bayminer.com/affiliate.html
https://www.bayminer.com/
https://www.bayminer.com/home.html
https://www.bayminer.com/product.html
https://www.bayminer.com/register.html
https://www.bayminer.com//about.html
https://www.bayminer.com/app.html
https://www.bayminer.com/FAQ.html
https://www.bayminer.com/affiliate.html
https://bjmining.com/home.html
https://bjmining.com/login.html
https://bjmining.com/register.html
https://bjmining.com/app.html
https://bjmining.com/about.html
https://bjmining.com/affiliate.html
https://bjmining.com/
https://bjmining.com/xml/favicon.ico
https://www.bjmining.com/xml/favicon.ico
https://www.bjmining.com/
https://www.bjmining.com/home.html
https://www.bjmining.com/login.html
https://www.bjmining.com/register.html
https://www.bjmining.com/app.html
https://www.bjmining.com/about.html
https://www.bjmining.com/affiliate.html
https://www.rimining.com
https://www.rimining.com/home.html
https://www.rimining.com/login.html
https://www.rimining.com/register.html
https://www.rimining.com/app.html
https://www.rimining.com/blog.html
https://www.rimining.com/about.html
https://www.rimining.com/favicon.ico
https://ourcryptominer.com
https://ourcryptominer.com/favicon.ico
https://ourcryptominer.com/home.html
https://ourcryptominer.com/login.html
https://ourcryptominer.com/register.html
https://ourcryptominer.com/app.html
https://ourcryptominer.com/about.html
https://ourcryptominer.com/blog.html
https://www.ourcryptominer.com
https://www.ourcryptominer.com/favicon.ico
https://www.ourcryptominer.com/home.html
https://www.ourcryptominer.com/login.html
https://www.ourcryptominer.com/register.html
https://www.ourcryptominer.com/app.html
https://www.ourcryptominer.com/about.html
https://www.ourcryptominer.com/blog.html
https://bayminer.com/
https://bayminer.com/home.html
https://bayminer.com/product.html
https://bayminer.com/register.html
https://bayminer.com//about.html
https://bayminer.com/app.html
https://bayminer.com/FAQ.html
https://bayminer.com/affiliate.html
https://www.bayminer.com/
https://www.bayminer.com/home.html
https://www.bayminer.com/product.html
https://www.bayminer.com/register.html
https://www.bayminer.com//about.html
https://www.bayminer.com/app.html
https://www.bayminer.com/FAQ.html
https://www.bayminer.com/affiliate.html
https://bjmining.com/home.html
https://bjmining.com/login.html
https://bjmining.com/register.html
https://bjmining.com/app.html
https://bjmining.com/about.html
https://bjmining.com/affiliate.html
https://bjmining.com/
https://bjmining.com/xml/favicon.ico
https://www.bjmining.com/xml/favicon.ico
https://www.bjmining.com/
https://www.bjmining.com/home.html
https://www.bjmining.com/login.html
https://www.bjmining.com/register.html
https://www.bjmining.com/app.html
https://www.bjmining.com/about.html
https://www.bjmining.com/affiliate.html
https://nr7miner.com/
https://www.rimining.com
https://www.rimining.com/home.html
https://www.rimining.com/login.html
https://www.rimining.com/register.html
https://www.rimining.com/app.html
https://www.rimining.com/blog.html
https://www.rimining.com/about.html
https://www.rimining.com/favicon.ico
https://gmominer.com
https://gmominer.com/xml/favicon.ico
https://gmominer.com/home.html
https://gmominer.com/product.html
https://gmominer.com/register.html
https://gmominer.com/about.html
https://gmominer.com/FAQ.html
https://gmominer.com/app.html
https://gmominer.com/teach.html
https://gmominer.com/affiliate.html
https://gmominer.com/welfare.html
https://www.gmominer.com
https://www.gmominer.com/xml/favicon.ico
https://www.gmominer.com/home.html
https://www.gmominer.com/product.html
https://www.gmominer.com/register.html
https://www.gmominer.com/about.html
https://www.gmominer.com/FAQ.html
https://www.gmominer.com/app.html
https://www.gmominer.com/teach.html
https://www.gmominer.com/affiliate.html
https://www.gmominer.com/welfare.html
https://nr7miner.com/
https://optominer.com/favicon.ico
https://optominer.com
https://optominer.com/home.html
https://optominer.com/login.html
https://optominer.com/register.html
https://optominer.com/app.html
https://optominer.com/blog.html
https://optominer.com/about.html
https://www.optominer.com/favicon.ico
https://www.optominer.com
https://www.optominer.com/home.html
https://www.optominer.com/login.html
https://www.optominer.com/register.html
https://www.optominer.com/app.html
https://www.optominer.com/blog.html
https://www.optominer.com/about.html
https://bayminer.com/
https://bayminer.com/home.html
https://bayminer.com/product.html
https://bayminer.com/register.html
https://bayminer.com//about.html
https://bayminer.com/app.html
https://bayminer.com/FAQ.html
https://bayminer.com/affiliate.html
https://www.bayminer.com/
https://www.bayminer.com/home.html
https://www.bayminer.com/product.html
https://www.bayminer.com/register.html
https://www.bayminer.com//about.html
https://www.bayminer.com/app.html
https://www.bayminer.com/FAQ.html
https://www.bayminer.com/affiliate.html
https://bjmining.com/home.html
https://bjmining.com/login.html
https://bjmining.com/register.html
https://bjmining.com/app.html
https://bjmining.com/about.html
https://bjmining.com/affiliate.html
https://bjmining.com/
https://bjmining.com/xml/favicon.ico
https://www.bjmining.com/xml/favicon.ico
https://www.bjmining.com/
https://www.bjmining.com/home.html
https://www.bjmining.com/login.html
https://www.bjmining.com/register.html
https://www.bjmining.com/app.html
https://www.bjmining.com/about.html
https://www.bjmining.com/affiliate.html
https://www.rimining.com
https://www.rimining.com/home.html
https://www.rimining.com/login.html
https://www.rimining.com/register.html
https://www.rimining.com/app.html
https://www.rimining.com/blog.html
https://www.rimining.com/about.html
https://www.rimining.com/favicon.ico
https://nr7miner.com/
https://www.nr7miner.com/
https://nr7miner.com/
https://dealmining.com/home.html
https://dealmining.com/login.html
https://dealmining.com/register.html
https://dealmining.com/about.html
https://dealmining.com/app.html
https://dealmining.com/FAQ.html
https://dealmining.com
https://www.dealmining.com/home.html
https://www.dealmining.com/login.html
https://www.dealmining.com/register.html
https://www.dealmining.com/about.html
https://www.dealmining.com/app.html
https://www.dealmining.com/FAQ.html
https://www.dealmining.com
https://nr7miner.com/
https://www.nr7miner.com/
https://nr7miner.com/
https://gmominer.com
https://gmominer.com/xml/favicon.ico
https://gmominer.com/home.html
https://gmominer.com/product.html
https://gmominer.com/register.html
https://gmominer.com/about.html
https://gmominer.com/FAQ.html
https://gmominer.com/app.html
https://gmominer.com/teach.html
https://gmominer.com/affiliate.html
<ahref="https://disposablecarts.co.uk/product/acapulco-gold-strain/" rel="dofollow">Acapulco Gold Strain</a>
<ahref="https://disposablecarts.co.uk/product/afghani-strain/" rel="dofollow">Afghani strain</a>
<ahref="https://disposablecarts.co.uk/product/ak-47-strain/" rel="dofollow">AK 47 Strain</a>
<ahref="https://disposablecarts.co.uk/product/amnesia-haze-strain/" rel="dofollow">Amnesia Haze Strain</a>
<ahref="https://disposablecarts.co.uk/product/blue-gelato-strain/" rel="dofollow">Blue Gelato Strain</a>
<ahref="https://disposablecarts.co.uk/product/blueberry-muffin-strain/" rel="dofollow">Blueberry Muffin Strain</a>
<ahref="https://disposablecarts.co.uk/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://disposablecarts.co.uk/product/chemdawg-strain/" rel="dofollow">Chemdawg Strain</a>
<ahref="https://disposablecarts.co.uk/product/diamond-dust-strain/" rel="dofollow">Diamond Dust Strain</a>
<ahref="https://disposablecarts.co.uk/product/gary-payton-strain/" rel="dofollow">Gary Payton Strain</a>
<ahref="https://disposablecarts.co.uk/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://disposablecarts.co.uk/product/og-kush/" rel="dofollow">OG Kush</a>
<ahref="https://disposablecarts.co.uk/product/permanent-marker-strain/" rel="dofollow">Permanent Marker Strain</a>
<ahref="https://disposablecarts.co.uk/product/zoapscotti-strain/" rel="dofollow">Zoapscotti Strain</a>
<ahref="https://disposablecarts.co.uk/product/blueberry-oil/" rel="dofollow">Blueberry Oil</a>
<ahref="https://disposablecarts.co.uk/product/co%e2%82%82-extract-oil/" rel="dofollow">CO₂ Extract Oil</a>
<ahref="https://disposablecarts.co.uk/product/delta-8-distillate/" rel="dofollow">Delta 8 Distillate</a>
<ahref="https://disposablecarts.co.uk/product/d9-distillate/" rel="dofollow">Delta 9 Distillate</a>
<ahref="https://disposablecarts.co.uk/product/delta-10-distillate/" rel="dofollow">DELTA-10 Distillate</a>
<ahref="https://disposablecarts.co.uk/product/hhc-distillate/" rel="dofollow">HHC Distillate</a>
<ahref="https://disposablecarts.co.uk/product/live-resin-oil/" rel="dofollow">Live Resin Oil</a>
<ahref="https://disposablecarts.co.uk/product/purple-punch-oil/" rel="dofollow">Purple Punch oil</a>
<ahref="https://disposablecarts.co.uk/product/raw-pure-thc-oil/" rel="dofollow">Raw (Pure) THC Oil</a>
<ahref="https://disposablecarts.co.uk/product/rick-simpson-oil/" rel="dofollow">Rick Simpson Oil</a>
<ahref="https://disposablecarts.co.uk/product/thc-clear-distillate/" rel="dofollow">THC Clear Distillate</a>
<ahref="https://disposablecarts.co.uk/product/thc-d9-syrup/" rel="dofollow">THC Delta 9 Syrup</a>
<ahref="https://disposablecarts.co.uk/product/star-killer-oil/" rel="dofollow">THC Star Killer Oil</a>
<ahref="https://disposablecarts.co.uk/product/thca-distillate/" rel="dofollow">THCA Distillate</a>
<ahref="https://disposablecarts.co.uk/product/thcp-distillate-oil/" rel="dofollow">THCP Distillate Oil</a>
<ahref="https://disposablecarts.co.uk/product/whole-melt-extracts-2g-disposable/" rel="dofollow">Whole Melt Extracts 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/tre-house-hhc-live-resin-disposable-vape-pens-2grams/" rel="dofollow">Tre House HHC Live Resin Disposable Vape Pens (2grams)</a>
<ahref="https://disposablecarts.co.uk/product/the-wizard-of-terps-1ml-syringe/" rel="dofollow">The Wizard Of Terps 1ml Syringe</a>
<ahref="https://disposablecarts.co.uk/product/ruby-carts-disposable-vape-pen/" rel="dofollow">Ruby Carts Disposable Vape Pen</a>
<ahref="https://disposablecarts.co.uk/product/potent-disposable/" rel="dofollow">Potent disposable</a>
<ahref="https://disposablecarts.co.uk/product/packwoods-x-runtz/" rel="dofollow">Packwoods x Runtz</a>
<ahref="https://disposablecarts.co.uk/product/ace-ultra-premium-disposable/" rel="dofollow">Ace Ultra Premium Disposable</a>
<ahref="https://disposablecarts.co.uk/product/backpackboyz-carts/" rel="dofollow">Backpackboyz Carts</a>
<ahref="https://disposablecarts.co.uk/product/baked-bar-2g-disposable/" rel="dofollow">Baked Bar 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/burst-2g-disposable-vape/" rel="dofollow">Burst 2g Disposable Vape</a>
<ahref="https://disposablecarts.co.uk/product/cali-company-disposable-vape/" rel="dofollow">Cali Company Disposable Vape</a>
<ahref="https://disposablecarts.co.uk/product/choice-lab-2g-disposable/" rel="dofollow">Choice Lab 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/clean-carts-2g-disposable/" rel="dofollow">Clean Carts 2G Disposable</a>
<ahref="https://disposablecarts.co.uk/product/cookies-1g-disposable/" rel="dofollow">Cookies 1g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/family-high-range/" rel="dofollow">Family High Range</a>
<ahref="https://disposablecarts.co.uk/product/favorites-2g-disposable/" rel="dofollow">Favorites 2G Disposable</a>
<ahref="https://disposablecarts.co.uk/product/gassed-up-2g-disposable/" rel="dofollow">Gassed Up 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/jeeter-juice-carts/" rel="dofollow">Jeeter Juice Carts</a>
<ahref="https://disposablecarts.co.uk/product/jungle-boys-vape/" rel="dofollow">Jungle Boys Vape</a>
<ahref="https://disposablecarts.co.uk/product/seedless-disposable/" rel="dofollow">Seedless Disposable</a>
<ahref="https://englandtrap.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://englandtrap.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://englandtrap.com/product/blueberry-zkittlez-strain/" rel="dofollow">Blueberry Strain</a>
<ahref="https://englandtrap.com/product/cheese-kush/" rel="dofollow">Cheese strain</a>
<ahref="https://englandtrap.com/product/gelato-strain/" rel="dofollow">Gelato Strain</a>
<ahref="https://englandtrap.com/product/gg4-strain/" rel="dofollow">GG4 Strain</a>
<ahref="https://englandtrap.com/product/godfather-og-strain/" rel="dofollow">Godfather OG Strain</a>
<ahref="https://englandtrap.com/product/stardawg-strain/" rel="dofollow">Stardawg Strain</a>
<ahref="https://englandtrap.com/product/super-lemon-cherry-strain/" rel="dofollow">Super Lemon Cherry Strain</a>
<ahref="https://englandtrap.com/product/wedding-cake-strain-2/" rel="dofollow">Wedding Cake Strain</a>
<ahref="https://englandtrap.com/product/white-widow-strain/" rel="dofollow">White Widow Strain</a>
<ahref="https://englandtrap.com/product/girl-scout-cookies/" rel="dofollow">Girl Scout Cookies</a>
<ahref="https://englandtrap.com/product/granddaddy-purple-gdp-strain/" rel="dofollow">Granddaddy Purple Strain</a>
<ahref="https://englandtrap.com/product/ice-cream-cake-strain/" rel="dofollow">Ice Cream Cake Strain</a>
<ahref="https://englandtrap.com/product/master-kush/" rel="dofollow">Master Kush</a>
<ahref="https://englandtrap.com/product/northern-lights/" rel="dofollow">Northern Lights strain</a>
<ahref="https://englandtrap.com/product/skywalker-kush/" rel="dofollow">Skywalker Kush</a>
<ahref="https://englandtrap.com/product/watermelon-gelato-strain/" rel="dofollow">Watermelon Gelato Strain</a>
<ahref="https://englandtrap.com/product/zeus-og-strain/" rel="dofollow">Zeus OG Strain</a>
<ahref="https://englandtrap.com/product/amnesia-haze/" rel="dofollow">Amnesia Haze</a>
<ahref="https://englandtrap.com/product/bruce-banner-strain/" rel="dofollow">Bruce Banner Strain</a>
<ahref="https://englandtrap.com/product/durban-poison/" rel="dofollow">Durban Poison</a>
<ahref="https://englandtrap.com/product/guava-weed-strain/" rel="dofollow">Guava Weed Strain</a>
<ahref="https://englandtrap.com/product/maui-wowie-strain/" rel="dofollow">Maui Wowie Strain</a>
<ahref="https://englandtrap.com/product/mimosa-strain/" rel="dofollow">Mimosa Strain</a>
<ahref="https://englandtrap.com/product/sour-diesel-strain/" rel="dofollow">Sour Diesel Strain</a>
<ahref="https://englandtrap.com/product/super-silver-haze/" rel="dofollow">Super Silver Haze</a>
<ahref="https://englandtrap.com/product/big-chief-carts/" rel="dofollow">Big Chief Carts</a>
<ahref="https://englandtrap.com/product/cake-vape-pen/" rel="dofollow">CAKE VAPE PEN</a>
<ahref="https://englandtrap.com/product/cali-company-1g-vapes/" rel="dofollow">Cali Company 1g Vapes</a>
<ahref="https://englandtrap.com/product/dope-disposable-vape-pens/" rel="dofollow">Dope Disposable Vape Pens</a>
<ahref="https://englandtrap.com/product/england-trap-1g-vape/" rel="dofollow">England Trap 1g Vape</a>
<ahref="https://englandtrap.com/product/expensive-sht-vapes/" rel="dofollow">Expensive Sh*t Vapes</a>
<ahref="https://englandtrap.com/product/fryd-vape/" rel="dofollow">FRYD VAPE</a>
<ahref="https://englandtrap.com/product/muha-meds-vape/" rel="dofollow">Muha Meds Vape</a>
<ahref="https://englandtrap.com/product/packman-vape/" rel="dofollow">PACKMAN VAPE</a>
<ahref="https://englandtrap.com/product/packwoods-vape/" rel="dofollow">PACKWOODS VAPE</a>
<ahref="https://hashclinicc.com/product/alien-og-hash/" rel="dofollow">Alien OG hash</a>
<ahref="https://hashclinicc.com/product/cali-plates-hash/" rel="dofollow">Cali Plates Hash</a>
<ahref="https://hashclinicc.com/product/fly-farm-hash/" rel="dofollow">FLY FARM HASH</a>
<ahref="https://hashclinicc.com/product/kilogrammes-farm-hash/" rel="dofollow">Kilogrammes Farm Hash</a>
<ahref="https://hashclinicc.com/product/la-mousse-hash/" rel="dofollow">La Mousse Hash</a>
<ahref="https://hashclinicc.com/product/lemon-haze-hash/" rel="dofollow">LEMON HAZE HASH</a>
<ahref="https://hashclinicc.com/product/pollen-hash/" rel="dofollow">POLLEN HASH</a>
<ahref="https://hashclinicc.com/product/static-room-hash/" rel="dofollow">Static Room Hash</a>
<ahref="https://hashclinicc.com/product/tangie-hash/" rel="dofollow">TANGIE HASH</a>
<ahref="https://hashclinicc.com/product/wazabi-hash/" rel="dofollow">WAZABI HASH</a>
<ahref="https://hashclinicc.com/product/41-unicornz-strain/" rel="dofollow">41 Unicornz Strain</a>
<ahref="https://hashclinicc.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://hashclinicc.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://hashclinicc.com/product/blue-gelato-strain/" rel="dofollow">Blue Gelato Strain</a>
<ahref="https://hashclinicc.com/product/blueberry-muffin-strain/" rel="dofollow">Blueberry Muffin Strain</a>
<ahref="https://hashclinicc.com/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://hashclinicc.com/product/gary-payton-strain/" rel="dofollow">Gary Payton Strain</a>
<ahref="https://hashclinicc.com/product/hella-jelly-strain/" rel="dofollow">Hella Jelly Strain</a>
<ahref="https://hashclinicc.com/product/kosher-kush-strain/" rel="dofollow">Kosher Kush Strain</a>
<ahref="https://hashclinicc.com/product/lava-cake-strain/" rel="dofollow">Lava Cake Strain</a>
<ahref="https://hashclinicc.com/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://hashclinicc.com/product/moonrock-pre-roll/" rel="dofollow">Moonrock Pre Roll</a>
<ahref="https://hashclinicc.com/product/og-kush/" rel="dofollow">OG Kush</a>
<ahref="https://hashclinicc.com/product/tropical-runtz-strain/" rel="dofollow">Tropical runtz strain</a>
<ahref="https://hashclinicc.com/product/watermelon-runtz-strain/" rel="dofollow">Watermelon Runtz Strain</a>
<ahref="https://hashclinicc.com/product/backpackboyz-cart/" rel="dofollow">Backpackboyz Cart</a>
<ahref="https://hashclinicc.com/product/baked-bar-disposable/" rel="dofollow">Baked Bar Disposable</a>
<ahref="https://hashclinicc.com/product/cali-plug-disposable-vape/" rel="dofollow">Cali Plug Disposable Vape</a>
<ahref="https://hashclinicc.com/product/clean-carts-2g-disposable/" rel="dofollow">Clean Carts 2G Disposable</a>
<ahref="https://hashclinicc.com/product/d9-distillate/" rel="dofollow">D9 Distillate</a>
<ahref="https://hashclinicc.com/product/elites-switch-1g-disposable/" rel="dofollow">Elites Switch 1g Disposable</a>
<ahref="https://hashclinicc.com/product/expensive-shit-1g-vape/" rel="dofollow">Expensive Shit 1g Vape</a>
<ahref="https://hashclinicc.com/product/fryd-donuts-disposable-2g/" rel="dofollow">Fryd Donuts Disposable (2g)</a>
<ahref="https://hashclinicc.com/product/gassed-up-2g-disposable/" rel="dofollow">Gassed Up 2g Disposable</a>
<ahref="https://hashclinicc.com/product/jungle-boys-vape/" rel="dofollow">Jungle Boys Vape</a>
<ahref="https://hashclinicc.com/product/kushy-punch-disposable/" rel="dofollow">Kushy Punch Disposable</a>
<ahref="https://hashclinicc.com/product/melt-x-packwoods/" rel="dofollow">Melt X Packwoods</a>
<ahref="https://hashclinicc.com/product/packspod-vape/" rel="dofollow">Packspod Vape</a>
<ahref="https://hashclinicc.com/product/the-cali-company-disposable-vape/" rel="dofollow">The Cali company disposable vape</a>
<ahref="https://hashclinicc.com/product/tyson-pod-1000mg/" rel="dofollow">Tyson Pod 1000mg</a>
<ahref="https://vapestorecart.com/product/buy-the-10-10-boys-2g-disposable-online/" rel "dofollow">Buy THE 10/10 BOYS 2G DISPOSABLE online</a>
<ahref="https://vapestorecart.com/product/buy-co2-extract-oil-online/" rel "dofollow">Buy CO2 Extract Oil Online</a>
<ahref="https://vapestorecart.com/product/buy-deep-sleep-thc-tincture-online/" rel "dofollow">Buy Deep Sleep THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-thc-mood-magic-tincture-online/" rel "dofollow">Buy THC Mood Magic Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-atlrx-delta-8-thc-online/" rel "dofollow">Buy ATLRx Delta 8 THC Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-watermelon-thc-tincture-online/" rel "dofollow">Buy Revival Watermelon THC Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-diamond-extracts-thc-tinctures-online/" rel "dofollow">Buy Diamond Extracts THC Tinctures Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-peach-thc-tincture-online/" rel "dofollow">Buy Revival Peach THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-terra-tonic-thc-tincture-d9-online/" rel "dofollow">Buy Terra Tonic THC Tincture D9 online</a>
<ahref="https://vapestorecart.com/product/buy-fryd-disposables-online/" rel "dofollow">Buy Fryd Disposables online</a>s
<ahref="https://vapestorecart.com/product/buy-smashed-disposables-vape/" rel "dofollow">Buy Smashed Disposables vape</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series-2/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/product/thc-p-vape-cartridge/" rel "dofollow">THC-P Vape Cartridge</a>
<ahref="https://vapestorecart.com/product/thca-disposable-2-gram-gourmet-desserts/" rel "dofollow">THCA Disposable 2 Gram – Gourmet Desserts</a>
<ahref="https://vapestorecart.com/product/blazed-7-gram-thca-delta-9b-disposable-vape/" rel "dofollow">Blazed 7 Gram THCA + Delta 9B Disposable Vape</a>
<ahref="https://vapestorecart.com/product/7-gram-thca-high-thc-p-disposable-vape-limited-series/" rel "dofollow">7 Gram THCA + High THC-P Disposable Vape – Limited Series</a>
<ahref="https://vapestorecart.com/product/knockout-blend-live-resin-disposable-2-gram/" rel "dofollow">Knockout Blend Live Resin Disposable – 2 Gram</a>
<ahref="https://vapestorecart.com/product/phc-disposable-vape-2-gram/" rel "dofollow">PHC Disposable Vape – 2 Gram</a>
<ahref="https://vapestorecart.com/product/hhc-disposable-vape-2-gram-tre-house/" rel "dofollow">HHC Disposable Vape 2 Gram – TRE House</a>
<ahref="https://vapestorecart.com/product/live-resin-hhc-p-disposable-vape-delta-extrax/" rel "dofollow">Live Resin HHC-P Disposable Vape – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thc-jd-3-gram-disposable-thins-delta-extrax/" rel "dofollow">THC-JD 3 Gram Disposable Thins – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thca-delta-9p-3-gram-disposable-blazed/" rel "dofollow">THCA + Delta 9P 3 Gram Disposable – Blazed</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/?post_type=product&p=519&preview=true" rel "dofollow">2 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/thca-vape-cartridge-exotic-kush-live-rosin/" rel "dofollow">THCA Vape Cartridge Exotic Kush – Live Rosin</a>
<ahref="https://vapestorecart.com/product/1-gram-thca-disposable-vapes-live-rosin/" rel "dofollow">1 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/2g-thca-disposable-vapes-minnesota-fire/" rel "dofollow">2G THCA Disposable Vapes – Minnesota Fire</a>
<ahref="https://vapestorecart.com/product/thc-vape-juice-wedding-cake/" rel "dofollow">THC Vape Juice Wedding Cake</a>
<ahref="https://vapestorecart.com/product/buy-urb-disposable-vape/" rel "dofollow">Buy URB Disposable vape</a>
<ahref="https://vapestorecart.com/product/buy-xtra-disposables-vape-online/" rel "dofollow">Buy Xtra Disposables vape online</a>
<ahref="https://vapestorecart.com/product/buy-runtz-disposable-vape-pen/" rel "dofollow">Buy RUNTZ DISPOSABLE VAPE PEN</a>
<ahref="https://vapestorecart.com/product/buy-dmt-vape-pens/" rel "dofollow">buy Dmt Vape Pens</a>
<ahref="https://vapestorecart.com/product/rainbows-and-cherries-live-budder/" rel "dofollow">Rainbows and Cherries Live Budder</a>
<ahref="https://vapestorecart.com/product/2-5g-citrus-mac-live-budder/" rel "dofollow">2.5g Citrus MAC Live Budder</a>
<ahref="https://vapestorecart.com/product/1g-peking-duck-live-sugar/" rel "dofollow">1g Peking Duck Live Sugar</a>
<ahref="https://vapestorecart.com/product/30ml-rest-15-tincture/" rel "dofollow">30ml Rest 1:5 Tincture</a>
<ahref="https://vapestorecart.com/product/1g-face-mints-liquid-live-resin-cartridge/" rel "dofollow">1g Face Mints Liquid Live Resin Cartridge</a>
<ahref="https://vapestorecart.com/product/acitivated-thc-distillate-strike-gold/" rel="dofollow">Activated THC distillate Strike gold</a>
<ahref="https://vapestorecart.com/product/platinum-cbd-oil-20-strength-10ml/" rel="dofollow">Platinum CBD Oil - 20% Strength (10ml)</a>
<ahref="https://vapestorecart.com/product/thc-oil-delta-8-thc-tincture-oil-1200mg/" rel="dofollow">THC Oil | Delta 8 THC Tincture Oil 1200mg</a>
<ahref="https://vapestorecart.com/product/delta-9-thc-oil-75mg-30ml-microdose/" rel="dofollow">Delta 9 THC Oil 75mg 30mL Microdose</a>
<ahref="https://vapestorecart.com/product/mile-high-aerovape-420-max/" rel="dofollow">Mile High Aerovape 420 Max</a>
<ahref="https://vapestorecart.com/product/urb-smart-device-thcap-disposable-6g/" rel="dofollow">Urb Smart Device THCAP Disposable 6g</a>
<ahref="https://vapestorecart.com/product/vape-pen-and-charger-with-cbd-cartridge/" rel="dofollow">Vape Pen and Charger with CBD Cartridge</a>
<ahref="https://vapestorecart.com/product/cbd-vape-pen-cartridges/" rel="dofollow">CBD Vape Pen Cartridges</a>
<ahref="https://vapestorecart.com/product/jet-fuel-1000mg/" rel="dofollow">Jet Fuel [1000mg]</a>
<ahref="https://disposablecarts.co.uk/product/acapulco-gold-strain/" rel="dofollow">Acapulco Gold Strain</a>
<ahref="https://disposablecarts.co.uk/product/afghani-strain/" rel="dofollow">Afghani strain</a>
<ahref="https://disposablecarts.co.uk/product/ak-47-strain/" rel="dofollow">AK 47 Strain</a>
<ahref="https://disposablecarts.co.uk/product/amnesia-haze-strain/" rel="dofollow">Amnesia Haze Strain</a>
<ahref="https://disposablecarts.co.uk/product/blue-gelato-strain/" rel="dofollow">Blue Gelato Strain</a>
<ahref="https://disposablecarts.co.uk/product/blueberry-muffin-strain/" rel="dofollow">Blueberry Muffin Strain</a>
<ahref="https://disposablecarts.co.uk/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://disposablecarts.co.uk/product/chemdawg-strain/" rel="dofollow">Chemdawg Strain</a>
<ahref="https://disposablecarts.co.uk/product/diamond-dust-strain/" rel="dofollow">Diamond Dust Strain</a>
<ahref="https://disposablecarts.co.uk/product/gary-payton-strain/" rel="dofollow">Gary Payton Strain</a>
<ahref="https://disposablecarts.co.uk/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://disposablecarts.co.uk/product/og-kush/" rel="dofollow">OG Kush</a>
<ahref="https://disposablecarts.co.uk/product/permanent-marker-strain/" rel="dofollow">Permanent Marker Strain</a>
<ahref="https://disposablecarts.co.uk/product/zoapscotti-strain/" rel="dofollow">Zoapscotti Strain</a>
<ahref="https://disposablecarts.co.uk/product/blueberry-oil/" rel="dofollow">Blueberry Oil</a>
<ahref="https://disposablecarts.co.uk/product/co%e2%82%82-extract-oil/" rel="dofollow">CO₂ Extract Oil</a>
<ahref="https://disposablecarts.co.uk/product/delta-8-distillate/" rel="dofollow">Delta 8 Distillate</a>
<ahref="https://disposablecarts.co.uk/product/d9-distillate/" rel="dofollow">Delta 9 Distillate</a>
<ahref="https://disposablecarts.co.uk/product/delta-10-distillate/" rel="dofollow">DELTA-10 Distillate</a>
<ahref="https://disposablecarts.co.uk/product/hhc-distillate/" rel="dofollow">HHC Distillate</a>
<ahref="https://disposablecarts.co.uk/product/live-resin-oil/" rel="dofollow">Live Resin Oil</a>
<ahref="https://disposablecarts.co.uk/product/purple-punch-oil/" rel="dofollow">Purple Punch oil</a>
<ahref="https://disposablecarts.co.uk/product/raw-pure-thc-oil/" rel="dofollow">Raw (Pure) THC Oil</a>
<ahref="https://disposablecarts.co.uk/product/rick-simpson-oil/" rel="dofollow">Rick Simpson Oil</a>
<ahref="https://disposablecarts.co.uk/product/thc-clear-distillate/" rel="dofollow">THC Clear Distillate</a>
<ahref="https://disposablecarts.co.uk/product/thc-d9-syrup/" rel="dofollow">THC Delta 9 Syrup</a>
<ahref="https://disposablecarts.co.uk/product/star-killer-oil/" rel="dofollow">THC Star Killer Oil</a>
<ahref="https://disposablecarts.co.uk/product/thca-distillate/" rel="dofollow">THCA Distillate</a>
<ahref="https://disposablecarts.co.uk/product/thcp-distillate-oil/" rel="dofollow">THCP Distillate Oil</a>
<ahref="https://disposablecarts.co.uk/product/whole-melt-extracts-2g-disposable/" rel="dofollow">Whole Melt Extracts 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/tre-house-hhc-live-resin-disposable-vape-pens-2grams/" rel="dofollow">Tre House HHC Live Resin Disposable Vape Pens (2grams)</a>
<ahref="https://disposablecarts.co.uk/product/the-wizard-of-terps-1ml-syringe/" rel="dofollow">The Wizard Of Terps 1ml Syringe</a>
<ahref="https://disposablecarts.co.uk/product/ruby-carts-disposable-vape-pen/" rel="dofollow">Ruby Carts Disposable Vape Pen</a>
<ahref="https://disposablecarts.co.uk/product/potent-disposable/" rel="dofollow">Potent disposable</a>
<ahref="https://disposablecarts.co.uk/product/packwoods-x-runtz/" rel="dofollow">Packwoods x Runtz</a>
<ahref="https://disposablecarts.co.uk/product/ace-ultra-premium-disposable/" rel="dofollow">Ace Ultra Premium Disposable</a>
<ahref="https://disposablecarts.co.uk/product/backpackboyz-carts/" rel="dofollow">Backpackboyz Carts</a>
<ahref="https://disposablecarts.co.uk/product/baked-bar-2g-disposable/" rel="dofollow">Baked Bar 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/burst-2g-disposable-vape/" rel="dofollow">Burst 2g Disposable Vape</a>
<ahref="https://disposablecarts.co.uk/product/cali-company-disposable-vape/" rel="dofollow">Cali Company Disposable Vape</a>
<ahref="https://disposablecarts.co.uk/product/choice-lab-2g-disposable/" rel="dofollow">Choice Lab 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/clean-carts-2g-disposable/" rel="dofollow">Clean Carts 2G Disposable</a>
<ahref="https://disposablecarts.co.uk/product/cookies-1g-disposable/" rel="dofollow">Cookies 1g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/family-high-range/" rel="dofollow">Family High Range</a>
<ahref="https://disposablecarts.co.uk/product/favorites-2g-disposable/" rel="dofollow">Favorites 2G Disposable</a>
<ahref="https://disposablecarts.co.uk/product/gassed-up-2g-disposable/" rel="dofollow">Gassed Up 2g Disposable</a>
<ahref="https://disposablecarts.co.uk/product/jeeter-juice-carts/" rel="dofollow">Jeeter Juice Carts</a>
<ahref="https://disposablecarts.co.uk/product/jungle-boys-vape/" rel="dofollow">Jungle Boys Vape</a>
<ahref="https://disposablecarts.co.uk/product/seedless-disposable/" rel="dofollow">Seedless Disposable</a>
<ahref="https://englandtrap.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://englandtrap.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://englandtrap.com/product/blueberry-zkittlez-strain/" rel="dofollow">Blueberry Strain</a>
<ahref="https://englandtrap.com/product/cheese-kush/" rel="dofollow">Cheese strain</a>
<ahref="https://englandtrap.com/product/gelato-strain/" rel="dofollow">Gelato Strain</a>
<ahref="https://englandtrap.com/product/gg4-strain/" rel="dofollow">GG4 Strain</a>
<ahref="https://englandtrap.com/product/godfather-og-strain/" rel="dofollow">Godfather OG Strain</a>
<ahref="https://englandtrap.com/product/stardawg-strain/" rel="dofollow">Stardawg Strain</a>
<ahref="https://englandtrap.com/product/super-lemon-cherry-strain/" rel="dofollow">Super Lemon Cherry Strain</a>
<ahref="https://englandtrap.com/product/wedding-cake-strain-2/" rel="dofollow">Wedding Cake Strain</a>
<ahref="https://englandtrap.com/product/white-widow-strain/" rel="dofollow">White Widow Strain</a>
<ahref="https://englandtrap.com/product/girl-scout-cookies/" rel="dofollow">Girl Scout Cookies</a>
<ahref="https://englandtrap.com/product/granddaddy-purple-gdp-strain/" rel="dofollow">Granddaddy Purple Strain</a>
<ahref="https://englandtrap.com/product/ice-cream-cake-strain/" rel="dofollow">Ice Cream Cake Strain</a>
<ahref="https://englandtrap.com/product/master-kush/" rel="dofollow">Master Kush</a>
<ahref="https://englandtrap.com/product/northern-lights/" rel="dofollow">Northern Lights strain</a>
<ahref="https://englandtrap.com/product/skywalker-kush/" rel="dofollow">Skywalker Kush</a>
<ahref="https://englandtrap.com/product/watermelon-gelato-strain/" rel="dofollow">Watermelon Gelato Strain</a>
<ahref="https://englandtrap.com/product/zeus-og-strain/" rel="dofollow">Zeus OG Strain</a>
<ahref="https://englandtrap.com/product/amnesia-haze/" rel="dofollow">Amnesia Haze</a>
<ahref="https://englandtrap.com/product/bruce-banner-strain/" rel="dofollow">Bruce Banner Strain</a>
<ahref="https://englandtrap.com/product/durban-poison/" rel="dofollow">Durban Poison</a>
<ahref="https://englandtrap.com/product/guava-weed-strain/" rel="dofollow">Guava Weed Strain</a>
<ahref="https://englandtrap.com/product/maui-wowie-strain/" rel="dofollow">Maui Wowie Strain</a>
<ahref="https://englandtrap.com/product/mimosa-strain/" rel="dofollow">Mimosa Strain</a>
<ahref="https://englandtrap.com/product/sour-diesel-strain/" rel="dofollow">Sour Diesel Strain</a>
<ahref="https://englandtrap.com/product/super-silver-haze/" rel="dofollow">Super Silver Haze</a>
<ahref="https://englandtrap.com/product/big-chief-carts/" rel="dofollow">Big Chief Carts</a>
<ahref="https://englandtrap.com/product/cake-vape-pen/" rel="dofollow">CAKE VAPE PEN</a>
<ahref="https://englandtrap.com/product/cali-company-1g-vapes/" rel="dofollow">Cali Company 1g Vapes</a>
<ahref="https://englandtrap.com/product/dope-disposable-vape-pens/" rel="dofollow">Dope Disposable Vape Pens</a>
<ahref="https://englandtrap.com/product/england-trap-1g-vape/" rel="dofollow">England Trap 1g Vape</a>
<ahref="https://englandtrap.com/product/expensive-sht-vapes/" rel="dofollow">Expensive Sh*t Vapes</a>
<ahref="https://englandtrap.com/product/fryd-vape/" rel="dofollow">FRYD VAPE</a>
<ahref="https://englandtrap.com/product/muha-meds-vape/" rel="dofollow">Muha Meds Vape</a>
<ahref="https://englandtrap.com/product/packman-vape/" rel="dofollow">PACKMAN VAPE</a>
<ahref="https://englandtrap.com/product/packwoods-vape/" rel="dofollow">PACKWOODS VAPE</a>
<ahref="https://hashclinicc.com/product/alien-og-hash/" rel="dofollow">Alien OG hash</a>
<ahref="https://hashclinicc.com/product/cali-plates-hash/" rel="dofollow">Cali Plates Hash</a>
<ahref="https://hashclinicc.com/product/fly-farm-hash/" rel="dofollow">FLY FARM HASH</a>
<ahref="https://hashclinicc.com/product/kilogrammes-farm-hash/" rel="dofollow">Kilogrammes Farm Hash</a>
<ahref="https://hashclinicc.com/product/la-mousse-hash/" rel="dofollow">La Mousse Hash</a>
<ahref="https://hashclinicc.com/product/lemon-haze-hash/" rel="dofollow">LEMON HAZE HASH</a>
<ahref="https://hashclinicc.com/product/pollen-hash/" rel="dofollow">POLLEN HASH</a>
<ahref="https://hashclinicc.com/product/static-room-hash/" rel="dofollow">Static Room Hash</a>
<ahref="https://hashclinicc.com/product/tangie-hash/" rel="dofollow">TANGIE HASH</a>
<ahref="https://hashclinicc.com/product/wazabi-hash/" rel="dofollow">WAZABI HASH</a>
<ahref="https://hashclinicc.com/product/41-unicornz-strain/" rel="dofollow">41 Unicornz Strain</a>
<ahref="https://hashclinicc.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://hashclinicc.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://hashclinicc.com/product/blue-gelato-strain/" rel="dofollow">Blue Gelato Strain</a>
<ahref="https://hashclinicc.com/product/blueberry-muffin-strain/" rel="dofollow">Blueberry Muffin Strain</a>
<ahref="https://hashclinicc.com/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://hashclinicc.com/product/gary-payton-strain/" rel="dofollow">Gary Payton Strain</a>
<ahref="https://hashclinicc.com/product/hella-jelly-strain/" rel="dofollow">Hella Jelly Strain</a>
<ahref="https://hashclinicc.com/product/kosher-kush-strain/" rel="dofollow">Kosher Kush Strain</a>
<ahref="https://hashclinicc.com/product/lava-cake-strain/" rel="dofollow">Lava Cake Strain</a>
<ahref="https://hashclinicc.com/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://hashclinicc.com/product/moonrock-pre-roll/" rel="dofollow">Moonrock Pre Roll</a>
<ahref="https://hashclinicc.com/product/og-kush/" rel="dofollow">OG Kush</a>
<ahref="https://hashclinicc.com/product/tropical-runtz-strain/" rel="dofollow">Tropical runtz strain</a>
<ahref="https://hashclinicc.com/product/watermelon-runtz-strain/" rel="dofollow">Watermelon Runtz Strain</a>
<ahref="https://hashclinicc.com/product/backpackboyz-cart/" rel="dofollow">Backpackboyz Cart</a>
<ahref="https://hashclinicc.com/product/baked-bar-disposable/" rel="dofollow">Baked Bar Disposable</a>
<ahref="https://hashclinicc.com/product/cali-plug-disposable-vape/" rel="dofollow">Cali Plug Disposable Vape</a>
<ahref="https://hashclinicc.com/product/clean-carts-2g-disposable/" rel="dofollow">Clean Carts 2G Disposable</a>
<ahref="https://hashclinicc.com/product/d9-distillate/" rel="dofollow">D9 Distillate</a>
<ahref="https://hashclinicc.com/product/elites-switch-1g-disposable/" rel="dofollow">Elites Switch 1g Disposable</a>
<ahref="https://hashclinicc.com/product/expensive-shit-1g-vape/" rel="dofollow">Expensive Shit 1g Vape</a>
<ahref="https://hashclinicc.com/product/fryd-donuts-disposable-2g/" rel="dofollow">Fryd Donuts Disposable (2g)</a>
<ahref="https://hashclinicc.com/product/gassed-up-2g-disposable/" rel="dofollow">Gassed Up 2g Disposable</a>
<ahref="https://hashclinicc.com/product/jungle-boys-vape/" rel="dofollow">Jungle Boys Vape</a>
<ahref="https://hashclinicc.com/product/kushy-punch-disposable/" rel="dofollow">Kushy Punch Disposable</a>
<ahref="https://hashclinicc.com/product/melt-x-packwoods/" rel="dofollow">Melt X Packwoods</a>
<ahref="https://hashclinicc.com/product/packspod-vape/" rel="dofollow">Packspod Vape</a>
<ahref="https://hashclinicc.com/product/the-cali-company-disposable-vape/" rel="dofollow">The Cali company disposable vape</a>
<ahref="https://hashclinicc.com/product/tyson-pod-1000mg/" rel="dofollow">Tyson Pod 1000mg</a>
<ahref="https://vapestorecart.com/product/buy-the-10-10-boys-2g-disposable-online/" rel "dofollow">Buy THE 10/10 BOYS 2G DISPOSABLE online</a>
<ahref="https://vapestorecart.com/product/buy-co2-extract-oil-online/" rel "dofollow">Buy CO2 Extract Oil Online</a>
<ahref="https://vapestorecart.com/product/buy-deep-sleep-thc-tincture-online/" rel "dofollow">Buy Deep Sleep THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-thc-mood-magic-tincture-online/" rel "dofollow">Buy THC Mood Magic Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-atlrx-delta-8-thc-online/" rel "dofollow">Buy ATLRx Delta 8 THC Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-watermelon-thc-tincture-online/" rel "dofollow">Buy Revival Watermelon THC Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-diamond-extracts-thc-tinctures-online/" rel "dofollow">Buy Diamond Extracts THC Tinctures Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-peach-thc-tincture-online/" rel "dofollow">Buy Revival Peach THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-terra-tonic-thc-tincture-d9-online/" rel "dofollow">Buy Terra Tonic THC Tincture D9 online</a>
<ahref="https://vapestorecart.com/product/buy-fryd-disposables-online/" rel "dofollow">Buy Fryd Disposables online</a>s
<ahref="https://vapestorecart.com/product/buy-smashed-disposables-vape/" rel "dofollow">Buy Smashed Disposables vape</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series-2/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/product/thc-p-vape-cartridge/" rel "dofollow">THC-P Vape Cartridge</a>
<ahref="https://vapestorecart.com/product/thca-disposable-2-gram-gourmet-desserts/" rel "dofollow">THCA Disposable 2 Gram – Gourmet Desserts</a>
<ahref="https://vapestorecart.com/product/blazed-7-gram-thca-delta-9b-disposable-vape/" rel "dofollow">Blazed 7 Gram THCA + Delta 9B Disposable Vape</a>
<ahref="https://vapestorecart.com/product/7-gram-thca-high-thc-p-disposable-vape-limited-series/" rel "dofollow">7 Gram THCA + High THC-P Disposable Vape – Limited Series</a>
<ahref="https://vapestorecart.com/product/knockout-blend-live-resin-disposable-2-gram/" rel "dofollow">Knockout Blend Live Resin Disposable – 2 Gram</a>
<ahref="https://vapestorecart.com/product/phc-disposable-vape-2-gram/" rel "dofollow">PHC Disposable Vape – 2 Gram</a>
<ahref="https://vapestorecart.com/product/hhc-disposable-vape-2-gram-tre-house/" rel "dofollow">HHC Disposable Vape 2 Gram – TRE House</a>
<ahref="https://vapestorecart.com/product/live-resin-hhc-p-disposable-vape-delta-extrax/" rel "dofollow">Live Resin HHC-P Disposable Vape – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thc-jd-3-gram-disposable-thins-delta-extrax/" rel "dofollow">THC-JD 3 Gram Disposable Thins – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thca-delta-9p-3-gram-disposable-blazed/" rel "dofollow">THCA + Delta 9P 3 Gram Disposable – Blazed</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/?post_type=product&p=519&preview=true" rel "dofollow">2 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/thca-vape-cartridge-exotic-kush-live-rosin/" rel "dofollow">THCA Vape Cartridge Exotic Kush – Live Rosin</a>
<ahref="https://vapestorecart.com/product/1-gram-thca-disposable-vapes-live-rosin/" rel "dofollow">1 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/2g-thca-disposable-vapes-minnesota-fire/" rel "dofollow">2G THCA Disposable Vapes – Minnesota Fire</a>
<ahref="https://vapestorecart.com/product/thc-vape-juice-wedding-cake/" rel "dofollow">THC Vape Juice Wedding Cake</a>
<ahref="https://vapestorecart.com/product/buy-urb-disposable-vape/" rel "dofollow">Buy URB Disposable vape</a>
<ahref="https://vapestorecart.com/product/buy-xtra-disposables-vape-online/" rel "dofollow">Buy Xtra Disposables vape online</a>
<ahref="https://vapestorecart.com/product/buy-runtz-disposable-vape-pen/" rel "dofollow">Buy RUNTZ DISPOSABLE VAPE PEN</a>
<ahref="https://vapestorecart.com/product/buy-dmt-vape-pens/" rel "dofollow">buy Dmt Vape Pens</a>
<ahref="https://vapestorecart.com/product/rainbows-and-cherries-live-budder/" rel "dofollow">Rainbows and Cherries Live Budder</a>
<ahref="https://vapestorecart.com/product/2-5g-citrus-mac-live-budder/" rel "dofollow">2.5g Citrus MAC Live Budder</a>
<ahref="https://vapestorecart.com/product/1g-peking-duck-live-sugar/" rel "dofollow">1g Peking Duck Live Sugar</a>
<ahref="https://vapestorecart.com/product/30ml-rest-15-tincture/" rel "dofollow">30ml Rest 1:5 Tincture</a>
<ahref="https://vapestorecart.com/product/1g-face-mints-liquid-live-resin-cartridge/" rel "dofollow">1g Face Mints Liquid Live Resin Cartridge</a>
<ahref="https://vapestorecart.com/product/acitivated-thc-distillate-strike-gold/" rel="dofollow">Activated THC distillate Strike gold</a>
<ahref="https://vapestorecart.com/product/platinum-cbd-oil-20-strength-10ml/" rel="dofollow">Platinum CBD Oil - 20% Strength (10ml)</a>
<ahref="https://vapestorecart.com/product/thc-oil-delta-8-thc-tincture-oil-1200mg/" rel="dofollow">THC Oil | Delta 8 THC Tincture Oil 1200mg</a>
<ahref="https://vapestorecart.com/product/delta-9-thc-oil-75mg-30ml-microdose/" rel="dofollow">Delta 9 THC Oil 75mg 30mL Microdose</a>
<ahref="https://vapestorecart.com/product/mile-high-aerovape-420-max/" rel="dofollow">Mile High Aerovape 420 Max</a>
<ahref="https://vapestorecart.com/product/urb-smart-device-thcap-disposable-6g/" rel="dofollow">Urb Smart Device THCAP Disposable 6g</a>
<ahref="https://vapestorecart.com/product/vape-pen-and-charger-with-cbd-cartridge/" rel="dofollow">Vape Pen and Charger with CBD Cartridge</a>
<ahref="https://vapestorecart.com/product/cbd-vape-pen-cartridges/" rel="dofollow">CBD Vape Pen Cartridges</a>
<ahref="https://vapestorecart.com/product/jet-fuel-1000mg/" rel="dofollow">Jet Fuel [1000mg]</a>
Superb postings. With thanks.
Amazing plenty of good information.
This was a great read—informative and easy to follow. Thanks for sharing such valuable content.
Very good
Amazing plenty of good information.
https://invromining.com/
https://invromining.com/xml/favicon.ico
https://comemining.com/xml/favicon.ico
https://comemining.com/
https://comemining.com/xml/favicon.ico
https://comemining.com/login.html
https://comemining.com/register.html
https://comemining.com/home.html
https://comemining.com/about.html
https://comemining.com/FAQ.html
https://comemining.com/app.html
https://gmominer.com
https://gmominer.com/xml/favicon.ico
Tips very well considered!
Very good
You’re a master of the written word!
Good stuff. Cheers!
<ahref="https://disposablecartsuk.com/product/acapulco-gold-strain/" rel="dofollow">Acapulco Gold Strain</a>
<ahref="https://disposablecartsuk.com/product/afghani-strain/" rel="dofollow">Afghani strain</a>
<ahref="https://disposablecartsuk.com/product/ak-47-strain/" rel="dofollow">AK 47 Strain</a>
<ahref="https://disposablecartsuk.com/product/amnesia-haze-strain/" rel="dofollow">Amnesia Haze Strain</a>
<ahref="https://disposablecartsuk.com/product/blue-gelato-strain/" rel="dofollow">Blue Gelato Strain</a>
<ahref="https://disposablecartsuk.com/product/blueberry-muffin-strain/" rel="dofollow">Blueberry Muffin Strain</a>
<ahref="https://disposablecartsuk.com/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://disposablecartsuk.com/product/chemdawg-strain/" rel="dofollow">Chemdawg Strain</a>
<ahref="https://disposablecartsuk.com/product/diamond-dust-strain/" rel="dofollow">Diamond Dust Strain</a>
<ahref="https://disposablecartsuk.com/product/gary-payton-strain/" rel="dofollow">Gary Payton Strain</a>
<ahref="https://disposablecartsuk.com/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://disposablecartsuk.com/product/og-kush/" rel="dofollow">OG Kush</a>
<ahref="https://disposablecartsuk.com/product/permanent-marker-strain/" rel="dofollow">Permanent Marker Strain</a>
<ahref="https://disposablecartsuk.com/product/zoapscotti-strain/" rel="dofollow">Zoapscotti Strain</a>
<ahref="https://disposablecartsuk.com/product/blueberry-oil/" rel="dofollow">Blueberry Oil</a>
<ahref="https://disposablecartsuk.com/product/co%e2%82%82-extract-oil/" rel="dofollow">CO₂ Extract Oil</a>
<ahref="https://disposablecartsuk.com/product/delta-8-distillate/" rel="dofollow">Delta 8 Distillate</a>
<ahref="https://disposablecartsuk.com/product/d9-distillate/" rel="dofollow">Delta 9 Distillate</a>
<ahref="https://disposablecartsuk.com/product/delta-10-distillate/" rel="dofollow">DELTA-10 Distillate</a>
<ahref="https://disposablecartsuk.com/product/hhc-distillate/" rel="dofollow">HHC Distillate</a>
<ahref="https://disposablecartsuk.com/product/live-resin-oil/" rel="dofollow">Live Resin Oil</a>
<ahref="https://disposablecartsuk.com/product/purple-punch-oil/" rel="dofollow">Purple Punch oil</a>
<ahref="https://disposablecartsuk.com/product/raw-pure-thc-oil/" rel="dofollow">Raw (Pure) THC Oil</a>
<ahref="https://disposablecartsuk.com/product/rick-simpson-oil/" rel="dofollow">Rick Simpson Oil</a>
<ahref="https://disposablecartsuk.com/product/thc-clear-distillate/" rel="dofollow">THC Clear Distillate</a>
<ahref="https://disposablecartsuk.com/product/thc-d9-syrup/" rel="dofollow">THC Delta 9 Syrup</a>
<ahref="https://disposablecartsuk.com/product/star-killer-oil/" rel="dofollow">THC Star Killer Oil</a>
<ahref="https://disposablecartsuk.com/product/thca-distillate/" rel="dofollow">THCA Distillate</a>
<ahref="https://disposablecartsuk.com/product/thcp-distillate-oil/" rel="dofollow">THCP Distillate Oil</a>
<ahref="https://disposablecartsuk.com/product/whole-melt-extracts-2g-disposable/" rel="dofollow">Whole Melt Extracts 2g Disposable</a>
<ahref="https://disposablecartsuk.com/product/tre-house-hhc-live-resin-disposable-vape-pens-2grams/" rel="dofollow">Tre House HHC Live Resin Disposable Vape Pens (2grams)</a>
<ahref="https://disposablecartsuk.com/product/the-wizard-of-terps-1ml-syringe/" rel="dofollow">The Wizard Of Terps 1ml Syringe</a>
<ahref="https://disposablecartsuk.com/product/ruby-carts-disposable-vape-pen/" rel="dofollow">Ruby Carts Disposable Vape Pen</a>
<ahref="https://disposablecartsuk.com/product/potent-disposable/" rel="dofollow">Potent disposable</a>
<ahref="https://disposablecartsuk.com/product/packwoods-x-runtz/" rel="dofollow">Packwoods x Runtz</a>
<ahref="https://disposablecartsuk.com/product/ace-ultra-premium-disposable/" rel="dofollow">Ace Ultra Premium Disposable</a>
<ahref="https://disposablecartsuk.com/product/backpackboyz-carts/" rel="dofollow">Backpackboyz Carts</a>
<ahref="https://disposablecartsuk.com/product/baked-bar-2g-disposable/" rel="dofollow">Baked Bar 2g Disposable</a>
<ahref="https://disposablecartsuk.com/product/burst-2g-disposable-vape/" rel="dofollow">Burst 2g Disposable Vape</a>
<ahref="https://disposablecartsuk.com/product/cali-company-disposable-vape/" rel="dofollow">Cali Company Disposable Vape</a>
<ahref="https://disposablecartsuk.com/product/choice-lab-2g-disposable/" rel="dofollow">Choice Lab 2g Disposable</a>
<ahref="https://disposablecartsuk.com/product/clean-carts-2g-disposable/" rel="dofollow">Clean Carts 2G Disposable</a>
<ahref="https://disposablecartsuk.com/product/cookies-1g-disposable/" rel="dofollow">Cookies 1g Disposable</a>
<ahref="https://disposablecartsuk.com/product/family-high-range/" rel="dofollow">Family High Range</a>
<ahref="https://disposablecartsuk.com/product/favorites-2g-disposable/" rel="dofollow">Favorites 2G Disposable</a>
<ahref="https://disposablecartsuk.com/product/gassed-up-2g-disposable/" rel="dofollow">Gassed Up 2g Disposable</a>
<ahref="https://disposablecartsuk.com/product/jeeter-juice-carts/" rel="dofollow">Jeeter Juice Carts</a>
<ahref="https://disposablecartsuk.com/product/jungle-boys-vape/" rel="dofollow">Jungle Boys Vape</a>
<ahref="https://disposablecartsuk.com/product/seedless-disposable/" rel="dofollow">Seedless Disposable</a>
<ahref="https://englandtrap.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://englandtrap.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://englandtrap.com/product/blueberry-zkittlez-strain/" rel="dofollow">Blueberry Strain</a>
<ahref="https://englandtrap.com/product/cheese-kush/" rel="dofollow">Cheese strain</a>
<ahref="https://englandtrap.com/product/gelato-strain/" rel="dofollow">Gelato Strain</a>
<ahref="https://englandtrap.com/product/gg4-strain/" rel="dofollow">GG4 Strain</a>
<ahref="https://englandtrap.com/product/godfather-og-strain/" rel="dofollow">Godfather OG Strain</a>
<ahref="https://englandtrap.com/product/stardawg-strain/" rel="dofollow">Stardawg Strain</a>
<ahref="https://englandtrap.com/product/super-lemon-cherry-strain/" rel="dofollow">Super Lemon Cherry Strain</a>
<ahref="https://englandtrap.com/product/wedding-cake-strain-2/" rel="dofollow">Wedding Cake Strain</a>
<ahref="https://englandtrap.com/product/white-widow-strain/" rel="dofollow">White Widow Strain</a>
<ahref="https://englandtrap.com/product/girl-scout-cookies/" rel="dofollow">Girl Scout Cookies</a>
<ahref="https://englandtrap.com/product/granddaddy-purple-gdp-strain/" rel="dofollow">Granddaddy Purple Strain</a>
<ahref="https://englandtrap.com/product/ice-cream-cake-strain/" rel="dofollow">Ice Cream Cake Strain</a>
<ahref="https://englandtrap.com/product/master-kush/" rel="dofollow">Master Kush</a>
<ahref="https://englandtrap.com/product/northern-lights/" rel="dofollow">Northern Lights strain</a>
<ahref="https://englandtrap.com/product/skywalker-kush/" rel="dofollow">Skywalker Kush</a>
<ahref="https://englandtrap.com/product/watermelon-gelato-strain/" rel="dofollow">Watermelon Gelato Strain</a>
<ahref="https://englandtrap.com/product/zeus-og-strain/" rel="dofollow">Zeus OG Strain</a>
<ahref="https://englandtrap.com/product/amnesia-haze/" rel="dofollow">Amnesia Haze</a>
<ahref="https://englandtrap.com/product/bruce-banner-strain/" rel="dofollow">Bruce Banner Strain</a>
<ahref="https://englandtrap.com/product/durban-poison/" rel="dofollow">Durban Poison</a>
<ahref="https://englandtrap.com/product/guava-weed-strain/" rel="dofollow">Guava Weed Strain</a>
<ahref="https://englandtrap.com/product/maui-wowie-strain/" rel="dofollow">Maui Wowie Strain</a>
<ahref="https://englandtrap.com/product/mimosa-strain/" rel="dofollow">Mimosa Strain</a>
<ahref="https://englandtrap.com/product/sour-diesel-strain/" rel="dofollow">Sour Diesel Strain</a>
<ahref="https://englandtrap.com/product/super-silver-haze/" rel="dofollow">Super Silver Haze</a>
<ahref="https://englandtrap.com/product/big-chief-carts/" rel="dofollow">Big Chief Carts</a>
<ahref="https://englandtrap.com/product/cake-vape-pen/" rel="dofollow">CAKE VAPE PEN</a>
<ahref="https://englandtrap.com/product/cali-company-1g-vapes/" rel="dofollow">Cali Company 1g Vapes</a>
<ahref="https://englandtrap.com/product/dope-disposable-vape-pens/" rel="dofollow">Dope Disposable Vape Pens</a>
<ahref="https://englandtrap.com/product/england-trap-1g-vape/" rel="dofollow">England Trap 1g Vape</a>
<ahref="https://englandtrap.com/product/expensive-sht-vapes/" rel="dofollow">Expensive Sh*t Vapes</a>
<ahref="https://englandtrap.com/product/fryd-vape/" rel="dofollow">FRYD VAPE</a>
<ahref="https://englandtrap.com/product/muha-meds-vape/" rel="dofollow">Muha Meds Vape</a>
<ahref="https://englandtrap.com/product/packman-vape/" rel="dofollow">PACKMAN VAPE</a>
<ahref="https://englandtrap.com/product/packwoods-vape/" rel="dofollow">PACKWOODS VAPE</a>
<ahref="https://englandtrap.com/product/cali-plates-hash/" rel="dofollow">Cali Plates Hash</a>
<ahref="https://englandtrap.com/product/fly-farm-hash/" rel="dofollow">FLY FARM HASH</a>
<ahref="https://englandtrap.com/product/16147/" rel="dofollow">Kilogrammes Farm Hash</a>
<ahref="https://englandtrap.com/product/la-mousse-hash-review/" rel="dofollow">La Mousse Hash</a>
<ahref="https://englandtrap.com/product/lemon-haze-hash/" rel="dofollow">LEMON HAZE HASH</a>
<ahref="https://englandtrap.com/product/static-room-hash/" rel="dofollow">Static Room Hash</a>
<ahref="https://englandtrap.com/product/super-pollen-hash/" rel="dofollow">Super Pollen Hash</a>
<ahref="https://englandtrap.com/product/tangie-hash/" rel="dofollow">TANGIE HASH</a>
<ahref="https://englandtrap.com/product/wazabi-hash/" rel="dofollow">WAZABI HASH</a>
<ahref="https://hashclinicc.com/product/alien-og-hash/" rel="dofollow">Alien OG hash</a>
<ahref="https://hashclinicc.com/product/cali-plates-hash/" rel="dofollow">Cali Plates Hash</a>
<ahref="https://hashclinicc.com/product/fly-farm-hash/" rel="dofollow">FLY FARM HASH</a>
<ahref="https://hashclinicc.com/product/kilogrammes-farm-hash/" rel="dofollow">Kilogrammes Farm Hash</a>
<ahref="https://hashclinicc.com/product/la-mousse-hash/" rel="dofollow">La Mousse Hash</a>
<ahref="https://hashclinicc.com/product/lemon-haze-hash/" rel="dofollow">LEMON HAZE HASH</a>
<ahref="https://hashclinicc.com/product/pollen-hash/" rel="dofollow">POLLEN HASH</a>
<ahref="https://hashclinicc.com/product/static-room-hash/" rel="dofollow">Static Room Hash</a>
<ahref="https://hashclinicc.com/product/tangie-hash/" rel="dofollow">TANGIE HASH</a>
<ahref="https://hashclinicc.com/product/wazabi-hash/" rel="dofollow">WAZABI HASH</a>
<ahref="https://hashclinicc.com/product/41-unicornz-strain/" rel="dofollow">41 Unicornz Strain</a>
<ahref="https://hashclinicc.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://hashclinicc.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://hashclinicc.com/product/blue-gelato-strain/" rel="dofollow">Blue Gelato Strain</a>
<ahref="https://hashclinicc.com/product/blueberry-muffin-strain/" rel="dofollow">Blueberry Muffin Strain</a>
<ahref="https://hashclinicc.com/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://hashclinicc.com/product/gary-payton-strain/" rel="dofollow">Gary Payton Strain</a>
<ahref="https://hashclinicc.com/product/hella-jelly-strain/" rel="dofollow">Hella Jelly Strain</a>
<ahref="https://hashclinicc.com/product/kosher-kush-strain/" rel="dofollow">Kosher Kush Strain</a>
<ahref="https://hashclinicc.com/product/lava-cake-strain/" rel="dofollow">Lava Cake Strain</a>
<ahref="https://hashclinicc.com/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://hashclinicc.com/product/moonrock-pre-roll/" rel="dofollow">Moonrock Pre Roll</a>
<ahref="https://hashclinicc.com/product/og-kush/" rel="dofollow">OG Kush</a>
<ahref="https://hashclinicc.com/product/tropical-runtz-strain/" rel="dofollow">Tropical runtz strain</a>
<ahref="https://hashclinicc.com/product/watermelon-runtz-strain/" rel="dofollow">Watermelon Runtz Strain</a>
<ahref="https://hashclinicc.com/product/backpackboyz-cart/" rel="dofollow">Backpackboyz Cart</a>
<ahref="https://hashclinicc.com/product/baked-bar-disposable/" rel="dofollow">Baked Bar Disposable</a>
<ahref="https://hashclinicc.com/product/cali-plug-disposable-vape/" rel="dofollow">Cali Plug Disposable Vape</a>
<ahref="https://hashclinicc.com/product/clean-carts-2g-disposable/" rel="dofollow">Clean Carts 2G Disposable</a>
<ahref="https://hashclinicc.com/product/d9-distillate/" rel="dofollow">D9 Distillate</a>
<ahref="https://hashclinicc.com/product/elites-switch-1g-disposable/" rel="dofollow">Elites Switch 1g Disposable</a>
<ahref="https://hashclinicc.com/product/expensive-shit-1g-vape/" rel="dofollow">Expensive Shit 1g Vape</a>
<ahref="https://hashclinicc.com/product/fryd-donuts-disposable-2g/" rel="dofollow">Fryd Donuts Disposable (2g)</a>
<ahref="https://hashclinicc.com/product/gassed-up-2g-disposable/" rel="dofollow">Gassed Up 2g Disposable</a>
<ahref="https://hashclinicc.com/product/jungle-boys-vape/" rel="dofollow">Jungle Boys Vape</a>
<ahref="https://hashclinicc.com/product/kushy-punch-disposable/" rel="dofollow">Kushy Punch Disposable</a>
<ahref="https://hashclinicc.com/product/melt-x-packwoods/" rel="dofollow">Melt X Packwoods</a>
<ahref="https://hashclinicc.com/product/packspod-vape/" rel="dofollow">Packspod Vape</a>
<ahref="https://hashclinicc.com/product/the-cali-company-disposable-vape/" rel="dofollow">The Cali company disposable vape</a>
<ahref="https://hashclinicc.com/product/tyson-pod-1000mg/" rel="dofollow">Tyson Pod 1000mg</a>
<ahref="https://vapestorecart.com/product/buy-the-10-10-boys-2g-disposable-online/" rel "dofollow">Buy THE 10/10 BOYS 2G DISPOSABLE online</a>
<ahref="https://vapestorecart.com/product/buy-co2-extract-oil-online/" rel "dofollow">Buy CO2 Extract Oil Online</a>
<ahref="https://vapestorecart.com/product/buy-deep-sleep-thc-tincture-online/" rel "dofollow">Buy Deep Sleep THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-thc-mood-magic-tincture-online/" rel "dofollow">Buy THC Mood Magic Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-atlrx-delta-8-thc-online/" rel "dofollow">Buy ATLRx Delta 8 THC Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-watermelon-thc-tincture-online/" rel "dofollow">Buy Revival Watermelon THC Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-diamond-extracts-thc-tinctures-online/" rel "dofollow">Buy Diamond Extracts THC Tinctures Online</a>
<ahref="https://vapestorecart.com/product/thc-p-vape-cartridge/" rel "dofollow">THC-P Vape Cartridge</a>
<ahref="https://vapestorecart.com/product/thca-disposable-2-gram-gourmet-desserts/" rel "dofollow">THCA Disposable 2 Gram – Gourmet Desserts</a>
<ahref="https://vapestorecart.com/product/blazed-7-gram-thca-delta-9b-disposable-vape/" rel "dofollow">Blazed 7 Gram THCA + Delta 9B Disposable Vape</a>
<ahref="https://vapestorecart.com/product/7-gram-thca-high-thc-p-disposable-vape-limited-series/" rel "dofollow">7 Gram THCA + High THC-P Disposable Vape – Limited Series</a>
<ahref="https://vapestorecart.com/product/knockout-blend-live-resin-disposable-2-gram/" rel "dofollow">Knockout Blend Live Resin Disposable – 2 Gram</a>
<ahref="https://vapestorecart.com/product/phc-disposable-vape-2-gram/" rel "dofollow">PHC Disposable Vape – 2 Gram</a>
<ahref="https://vapestorecart.com/product/hhc-disposable-vape-2-gram-tre-house/" rel "dofollow">HHC Disposable Vape 2 Gram – TRE House</a>
<ahref="https://vapestorecart.com/product/live-resin-hhc-p-disposable-vape-delta-extrax/" rel "dofollow">Live Resin HHC-P Disposable Vape – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thc-jd-3-gram-disposable-thins-delta-extrax/" rel "dofollow">THC-JD 3 Gram Disposable Thins – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thca-delta-9p-3-gram-disposable-blazed/" rel "dofollow">THCA + Delta 9P 3 Gram Disposable – Blazed</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/?post_type=product&p=519&preview=true" rel "dofollow">2 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/thca-vape-cartridge-exotic-kush-live-rosin/" rel "dofollow">THCA Vape Cartridge Exotic Kush – Live Rosin</a>
<ahref="https://vapestorecart.com/product/1-gram-thca-disposable-vapes-live-rosin/" rel "dofollow">1 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/2g-thca-disposable-vapes-minnesota-fire/" rel "dofollow">2G THCA Disposable Vapes – Minnesota Fire</a>
<ahref="https://vapestorecart.com/product/thc-vape-juice-wedding-cake/" rel "dofollow">THC Vape Juice Wedding Cake</a>
<ahref="https://vapestorecart.com/product/buy-urb-disposable-vape/" rel "dofollow">Buy URB Disposable vape</a>
<ahref="https://vapestorecart.com/product/buy-xtra-disposables-vape-online/" rel "dofollow">Buy Xtra Disposables vape online</a>
<ahref="https://vapestorecart.com/product/buy-runtz-disposable-vape-pen/" rel "dofollow">Buy RUNTZ DISPOSABLE VAPE PEN</a>
<ahref="https://vapestorecart.com/product/buy-dmt-vape-pens/" rel "dofollow">buy Dmt Vape Pens</a>
<ahref="https://vapestorecart.com/product/rainbows-and-cherries-live-budder/" rel "dofollow">Rainbows and Cherries Live Budder</a>
<ahref="https://vapestorecart.com/product/2-5g-citrus-mac-live-budder/" rel "dofollow">2.5g Citrus MAC Live Budder</a>
<ahref="https://vapestorecart.com/product/1g-peking-duck-live-sugar/" rel "dofollow">1g Peking Duck Live Sugar</a>
<ahref="https://vapestorecart.com/product/30ml-rest-15-tincture/" rel "dofollow">30ml Rest 1:5 Tincture</a>
<ahref="https://vapestorecart.com/product/1g-face-mints-liquid-live-resin-cartridge/" rel "dofollow">1g Face Mints Liquid Live Resin Cartridge</a>
<ahref="https://vapestorecart.com/product/acitivated-thc-distillate-strike-gold/" rel="dofollow">Activated THC distillate Strike gold</a>
<ahref="https://psychedelictripgateway.com/product/good-trip-mushroom-chocolate-bars/" rel="dofollow">Good Trip Chocolate Bars</a>
<ahref="https://psychedelictripgateway.com/product/fusion-mushroom-chocolate-bars/" rel="dofollow">Fusion Mushroom Chocolate Bars</a>
<ahref="https://psychedelictripgateway.com/product/lsd-acid/" rel="dofollow">Lsd (acid)</a>
<ahref="https://psychedelictripgateway.com/product/lsd-gel/" rel="dofollow">Lsd Gel Tabs</a>
<ahref="https://psychedelictripgateway.com/product/lsd-sheets/" rel="dofollow">LSD SHEETS</a>
<ahref="https://psychedelictripgateway.com/product/lsd/" rel="dofollow">Lsd</a>
<ahref="https://psychedelictripgateway.com/product/green-hulk-250mg-mdma/" rel="dofollow">Green Hulk (MDMA)</a>
<ahref="https://psychedelictripgateway.com/product/white-yellow-technogym/" rel="dofollow">White & Yellow Technogym</a>
<ahref="https://psychedelictripgateway.com/product/orange-sprite-mdma/" rel="dofollow">Orange Sprite MDMA Pills</a>
<ahref="https://psychedelictripgateway.com/product/red-bull-258mg-mdma/" rel="dofollow">Red Bull Pills (MDMA)</a>
<ahref="https://psychedelictripgateway.com/product/orange-trump-260mg-mdma/" rel="dofollow">Orange Trump 260mg MDMA</a>
<ahref="https://psychedelictripgateway.com/product/one-up-mushroom-chocolate-bars/" rel="dofollow">One Up Mushroom Chocolate Bars</a>
<ahref="https://psychedelictripgateway.com/product/one-up-raspberry-dark-chocolate-shroom-bars/" rel="dofollow">One Up Raspberry Dark Shroom Chocolate Bars</a>
<ahref="https://psychedelictripgateway.com/product/one-up-tagalongs-mushroom-bars/" rel="dofollow">One Up Tagalongs Mushroom Bars</a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-vanilla-biscuit/" rel="dofollow"></a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-vanilla-biscuit/" rel="dofollow">One up Multiverse Vanilla Biscuit</a>
<ahref="https://psychedelictripgateway.com/product/one-up-mushroom-bars-cookies-and-cream/" rel="dofollow">One Up Psilocybin Mushroom Cookies and Cream</a>
<ahref="https://psychedelictripgateway.com/product/one-up-mushroom-bars-do-si-dos/" rel="dofollow">One Up Do-Si-Dos Mushroom Bars</a>
<ahref="https://psychedelictripgateway.com/product/one-up-samoas-mushroom-bar/" rel="dofollow">One Up Samoas Mushroom Bar</a>
<ahref="https://psychedelictripgateway.com/product/one-up-strawberries-and-cream-mushroom-bars/" rel="dofollow">One Up Strawberries and Cream Mushroom Bars</a>
<ahref="https://psychedelictripgateway.com/product/one-up-thin-mints-mushroom-bars/" rel="dofollow">One Up Thin Mints Mushroom Bars</a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-almond-crush-chocolate-bar/" rel="dofollow">One Up Multiverse Almond Crush Chocolate Bar</a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-blueberry-yogurt/" rel="dofollow">One Up Multiverse Blueberry Yogurt</a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-oreo-milkshake/" rel="dofollow">One up Multiverse Oreo Milkshake</a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-matcha-milk-tea/" rel="dofollow">One up Multiverse Matcha Milk Tea</a>
<ahref="https://psychedelictripgateway.com/product/one-up-multiverse-strawberry-shortcake/" rel="dofollow">One up Multiverse Strawberry Shortcake</a>
<ahref="https://psychedelictripgateway.com/product/one-up-psychedelic-chocolate-bar/" rel="dofollow">One Up Psilocybin Chocolate Bar</a>
<ahref="https://psychedelictripgateway.com/product/one-up-trefoils-mushroom-bars/" rel="dofollow">One Up Trefoils Mushroom Bars</a>
<ahref="https://psychedelictripgateway.com/product/filthy-laboratory/" rel="dofollow">FILTHY LABORATORY</a>
<ahref="https://psychedelictripgateway.com/product/flavrx-cartridge/" rel="dofollow">FlavRX Cartridge</a>
<ahref="https://psychedelictripgateway.com/product/chronopoly-carts/" rel="dofollow">Chronopoly Carts</a>
<ahref="https://psychedelictripgateway.com/product/big-chief-extracts/" rel="dofollow">Big chief extracts</a>
<ahref="https://psychedelictripgateway.com/product/exxus-snap/" rel="dofollow">Exxus Snap</a>
<ahref="https://psychedelictripgateway.com/product/stiiizys-starter-kit/" rel="dofollow">Stiiizy Disposable</a>
<ahref="https://psychedelictripgateway.com/product/exotic-carts/" rel="dofollow">EXOTIC CARTS</a>
<ahref="https://psychedelictripgateway.com/product/brass-knuckles-cartridges/" rel="dofollow">Brass Knuckles Cartridges</a>
<ahref="https://psychedelictripgateway.com/product/5-me0-dmt/" rel="dofollow">5-Me0-DMT</a>
<ahref="https://psychedelictripgateway.com/product/albino-penis-envy-grow-kits/" rel="dofollow">albino penis envy grow kits</a>
<ahref="https://psychedelictripgateway.com/product/blue-oyster-mushrooms/" rel="dofollow">blue Oyster Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/lions-mane/" rel="dofollow">Lion’s Mane</a>
<ahref="https://psychedelictripgateway.com/product/dmt/" rel="dofollow">DMT</a>
<ahref="https://psychedelictripgateway.com/product/ecstasy-molly/" rel="dofollow">Ecstasy Pills</a>
<ahref="https://psychedelictripgateway.com/product/dmt-vape-pen-and-cartridges/" rel="dofollow">DMT VAPE PEN</a>
<ahref="https://psychedelictripgateway.com/product/5-meo-dmt/" rel="dofollow">5-MeO DMT</a>
<ahref="https://psychedelictripgateway.com/product/lsd-liquid/" rel="dofollow">Liquid LSD</a>
<ahref="https://psychedelictripgateway.com/product/gel-tabs-lsd-300ug/" rel="dofollow">Gel tabs lsd</a>
<ahref="https://psychedelictripgateway.com/product/lsd-tabs-250ug/" rel="dofollow">LSD Tabs</a>
<ahref="https://psychedelictripgateway.com/product/mdma-crystal/" rel="dofollow">MDMA Crystal</a>
<ahref="https://psychedelictripgateway.com/product/psilocybe-azurescens-dried-shrooms/" rel="dofollow">Psilocybe Azurescens mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/golden-teacher-mushrooms/" rel="dofollow">Golden Teacher Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/true-albino-teacher-mushroom/" rel="dofollow">True Albino Teacher</a>
<ahref="https://psychedelictripgateway.com/product/psilocybe-cyanescens-dried-shrooms/" rel="dofollow">Psilocybe Cyanescens</a>
<ahref="https://psychedelictripgateway.com/product/penis-envy/" rel="dofollow">Penis Envy</a>
<ahref="https://psychedelictripgateway.com/product/great-white-monster-psilocybe-cubensis/" rel="dofollow">Great White Monster-Psilocybe Cubensis</a>
<ahref="https://psychedelictripgateway.com/product/big-daddy-psilocybe-cubensis/" rel="dofollow">Big Daddy Psilocybe Cubensis</a>
<ahref="https://psychedelictripgateway.com/product/avery-albino-magic-mushroom/" rel="dofollow">Avery Albino Magic Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/albino-cambodians-psilocybe-cubensis/" rel="dofollow">Albino Cambodians Psilocybe Cubensis</a>
<ahref="https://psychedelictripgateway.com/product/albino-a-magic-mushroom-dried-shrooms/" rel="dofollow">Albino A+ Magic Mushroom</a>
<ahref="https://vapestorecart.com/product/buy-the-10-10-boys-2g-disposable-online/" rel "dofollow">Buy THE 10/10 BOYS 2G DISPOSABLE online</a>
<ahref="https://vapestorecart.com/product/buy-co2-extract-oil-online/" rel "dofollow">Buy CO2 Extract Oil Online</a>
<ahref="https://vapestorecart.com/product/buy-deep-sleep-thc-tincture-online/" rel "dofollow">Buy Deep Sleep THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-thc-mood-magic-tincture-online/" rel "dofollow">Buy THC Mood Magic Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-atlrx-delta-8-thc-online/" rel "dofollow">Buy ATLRx Delta 8 THC Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-watermelon-thc-tincture-online/" rel "dofollow">Buy Revival Watermelon THC Tincture online</a>
<ahref="https://vapestorecart.com/product/buy-diamond-extracts-thc-tinctures-online/" rel "dofollow">Buy Diamond Extracts THC Tinctures Online</a>
<ahref="https://vapestorecart.com/product/buy-revival-peach-thc-tincture-online/" rel "dofollow">Buy Revival Peach THC Tincture Online</a>
<ahref="https://vapestorecart.com/product/buy-terra-tonic-thc-tincture-d9-online/" rel "dofollow">Buy Terra Tonic THC Tincture D9 online</a>
<ahref="https://vapestorecart.com/product/buy-fryd-disposables-online/" rel "dofollow">Buy Fryd Disposables online</a>s
<ahref="https://vapestorecart.com/product/buy-smashed-disposables-vape/" rel "dofollow">Buy Smashed Disposables vape</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series-2/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/product/thc-p-vape-cartridge/" rel "dofollow">THC-P Vape Cartridge</a>
<ahref="https://vapestorecart.com/product/thca-disposable-2-gram-gourmet-desserts/" rel "dofollow">THCA Disposable 2 Gram – Gourmet Desserts</a>
<ahref="https://vapestorecart.com/product/blazed-7-gram-thca-delta-9b-disposable-vape/" rel "dofollow">Blazed 7 Gram THCA + Delta 9B Disposable Vape</a>
<ahref="https://vapestorecart.com/product/7-gram-thca-high-thc-p-disposable-vape-limited-series/" rel "dofollow">7 Gram THCA + High THC-P Disposable Vape – Limited Series</a>
<ahref="https://vapestorecart.com/product/knockout-blend-live-resin-disposable-2-gram/" rel "dofollow">Knockout Blend Live Resin Disposable – 2 Gram</a>
<ahref="https://vapestorecart.com/product/phc-disposable-vape-2-gram/" rel "dofollow">PHC Disposable Vape – 2 Gram</a>
<ahref="https://vapestorecart.com/product/hhc-disposable-vape-2-gram-tre-house/" rel "dofollow">HHC Disposable Vape 2 Gram – TRE House</a>
<ahref="https://vapestorecart.com/product/live-resin-hhc-p-disposable-vape-delta-extrax/" rel "dofollow">Live Resin HHC-P Disposable Vape – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thc-jd-3-gram-disposable-thins-delta-extrax/" rel "dofollow">THC-JD 3 Gram Disposable Thins – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thca-delta-9p-3-gram-disposable-blazed/" rel "dofollow">THCA + Delta 9P 3 Gram Disposable – Blazed</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/?post_type=product&p=519&preview=true" rel "dofollow">2 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/thca-vape-cartridge-exotic-kush-live-rosin/" rel "dofollow">THCA Vape Cartridge Exotic Kush – Live Rosin</a>
<ahref="https://vapestorecart.com/product/1-gram-thca-disposable-vapes-live-rosin/" rel "dofollow">1 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/2g-thca-disposable-vapes-minnesota-fire/" rel "dofollow">2G THCA Disposable Vapes – Minnesota Fire</a>
<ahref="https://vapestorecart.com/product/thc-vape-juice-wedding-cake/" rel "dofollow">THC Vape Juice Wedding Cake</a>
<ahref="https://vapestorecart.com/product/buy-urb-disposable-vape/" rel "dofollow">Buy URB Disposable vape</a>
<ahref="https://vapestorecart.com/product/buy-xtra-disposables-vape-online/" rel "dofollow">Buy Xtra Disposables vape online</a>
<ahref="https://vapestorecart.com/product/buy-runtz-disposable-vape-pen/" rel "dofollow">Buy RUNTZ DISPOSABLE VAPE PEN</a>
<ahref="https://vapestorecart.com/product/buy-dmt-vape-pens/" rel "dofollow">buy Dmt Vape Pens</a>
<ahref="https://vapestorecart.com/product/rainbows-and-cherries-live-budder/" rel "dofollow">Rainbows and Cherries Live Budder</a>
<ahref="https://vapestorecart.com/product/2-5g-citrus-mac-live-budder/" rel "dofollow">2.5g Citrus MAC Live Budder</a>
<ahref="https://vapestorecart.com/product/1g-peking-duck-live-sugar/" rel "dofollow">1g Peking Duck Live Sugar</a>
<ahref="https://vapestorecart.com/product/30ml-rest-15-tincture/" rel "dofollow">30ml Rest 1:5 Tincture</a>
<ahref="https://vapestorecart.com/product/1g-face-mints-liquid-live-resin-cartridge/" rel "dofollow">1g Face Mints Liquid Live Resin Cartridge</a>
<ahref="https://vapestorecart.com/product/acitivated-thc-distillate-strike-gold/" rel="dofollow">Activated THC distillate Strike gold</a>
<ahref="https://vapestorecart.com/product/platinum-cbd-oil-20-strength-10ml/" rel="dofollow">Platinum CBD Oil - 20% Strength (10ml)</a>
<ahref="https://vapestorecart.com/product/thc-oil-delta-8-thc-tincture-oil-1200mg/" rel="dofollow">THC Oil | Delta 8 THC Tincture Oil 1200mg</a>
<ahref="https://vapestorecart.com/product/delta-9-thc-oil-75mg-30ml-microdose/" rel="dofollow">Delta 9 THC Oil 75mg 30mL Microdose</a>
<ahref="https://vapestorecart.com/product/mile-high-aerovape-420-max/" rel="dofollow">Mile High Aerovape 420 Max</a>
<ahref="https://vapestorecart.com/product/urb-smart-device-thcap-disposable-6g/" rel="dofollow">Urb Smart Device THCAP Disposable 6g</a>
<ahref="https://vapestorecart.com/product/vape-pen-and-charger-with-cbd-cartridge/" rel="dofollow">Vape Pen and Charger with CBD Cartridge</a>
<ahref="https://vapestorecart.com/product/cbd-vape-pen-cartridges/" rel="dofollow">CBD Vape Pen Cartridges</a>
<ahref="https://vapestorecart.com/product/jet-fuel-1000mg/" rel="dofollow">Jet Fuel [1000mg]</a>
<ahref="https://vapestorecart.com/product/thc-flowers/" rel="dofollow">THC FLOWERS</a>
https://cmminer.com/
https://cmminer.com/
https://cpspai.com/
https://www.cpspai.com/
https://qfscoin.com
https://dnsbtc.com
https://www.qfscoin.com
https://www.dnsbtc.com
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series-2/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/product/thc-p-vape-cartridge/" rel "dofollow">THC-P Vape Cartridge</a>
<ahref="https://vapestorecart.com/product/thca-disposable-2-gram-gourmet-desserts/" rel "dofollow">THCA Disposable 2 Gram – Gourmet Desserts</a>
<ahref="https://vapestorecart.com/product/blazed-7-gram-thca-delta-9b-disposable-vape/" rel "dofollow">Blazed 7 Gram THCA + Delta 9B Disposable Vape</a>
<ahref="https://vapestorecart.com/product/7-gram-thca-high-thc-p-disposable-vape-limited-series/" rel "dofollow">7 Gram THCA + High THC-P Disposable Vape – Limited Series</a>
<ahref="https://vapestorecart.com/product/knockout-blend-live-resin-disposable-2-gram/" rel "dofollow">Knockout Blend Live Resin Disposable – 2 Gram</a>
<ahref="https://vapestorecart.com/product/phc-disposable-vape-2-gram/" rel "dofollow">PHC Disposable Vape – 2 Gram</a>
<ahref="https://vapestorecart.com/product/hhc-disposable-vape-2-gram-tre-house/" rel "dofollow">HHC Disposable Vape 2 Gram – TRE House</a>
<ahref="https://vapestorecart.com/product/live-resin-hhc-p-disposable-vape-delta-extrax/" rel "dofollow">Live Resin HHC-P Disposable Vape – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thc-jd-3-gram-disposable-thins-delta-extrax/" rel "dofollow">THC-JD 3 Gram Disposable Thins – Delta Extrax</a>
<ahref="https://vapestorecart.com/product/thca-delta-9p-3-gram-disposable-blazed/" rel "dofollow">THCA + Delta 9P 3 Gram Disposable – Blazed</a>
<ahref="https://vapestorecart.com/product/5-gram-thca-thcm-disposable-vape-exclusive-series/" rel "dofollow">5 Gram THCA + THCM Disposable Vape – Exclusive Series</a>
<ahref="https://vapestorecart.com/?post_type=product&p=519&preview=true" rel "dofollow">2 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/thca-vape-cartridge-exotic-kush-live-rosin/" rel "dofollow">THCA Vape Cartridge Exotic Kush – Live Rosin</a>
<ahref="https://vapestorecart.com/product/1-gram-thca-disposable-vapes-live-rosin/" rel "dofollow">1 Gram THCA Disposable Vapes – Live Rosin</a>
<ahref="https://vapestorecart.com/product/2g-thca-disposable-vapes-minnesota-fire/" rel "dofollow">2G THCA Disposable Vapes – Minnesota Fire</a>
<ahref="https://vapestorecart.com/product/thc-vape-juice-wedding-cake/" rel "dofollow">THC Vape Juice Wedding Cake</a>
<ahref="https://vapestorecart.com/product/buy-urb-disposable-vape/" rel "dofollow">Buy URB Disposable vape</a>
<ahref="https://vapestorecart.com/product/buy-xtra-disposables-vape-online/" rel "dofollow">Buy Xtra Disposables vape online</a>
<ahref="https://vapestorecart.com/product/buy-runtz-disposable-vape-pen/" rel "dofollow">Buy RUNTZ DISPOSABLE VAPE PEN</a>
<ahref="https://vapestorecart.com/product/buy-dmt-vape-pens/" rel "dofollow">buy Dmt Vape Pens</a>
<ahref="https://vapestorecart.com/product/rainbows-and-cherries-live-budder/" rel "dofollow">Rainbows and Cherries Live Budder</a>
<ahref="https://vapestorecart.com/product/2-5g-citrus-mac-live-budder/" rel "dofollow">2.5g Citrus MAC Live Budder</a>
<ahref="https://vapestorecart.com/product/1g-peking-duck-live-sugar/" rel "dofollow">1g Peking Duck Live Sugar</a>
<ahref="https://vapestorecart.com/product/30ml-rest-15-tincture/" rel "dofollow">30ml Rest 1:5 Tincture</a>
<ahref="https://vapestorecart.com/product/1g-face-mints-liquid-live-resin-cartridge/" rel "dofollow">1g Face Mints Liquid Live Resin Cartridge</a>
<ahref="https://vapestorecart.com/product/acitivated-thc-distillate-strike-gold/" rel="dofollow">Activated THC distillate Strike gold</a>
<ahref="https://vapestorecart.com/product/platinum-cbd-oil-20-strength-10ml/" rel="dofollow">Platinum CBD Oil - 20% Strength (10ml)</a>
<ahref="https://vapestorecart.com/product/thc-oil-delta-8-thc-tincture-oil-1200mg/" rel="dofollow">THC Oil | Delta 8 THC Tincture Oil 1200mg</a>
<ahref="https://vapestorecart.com/product/delta-9-thc-oil-75mg-30ml-microdose/" rel="dofollow">Delta 9 THC Oil 75mg 30mL Microdose</a>
<ahref="https://vapestorecart.com/product/mile-high-aerovape-420-max/" rel="dofollow">Mile High Aerovape 420 Max</a>
<ahref="https://vapestorecart.com/product/urb-smart-device-thcap-disposable-6g/" rel="dofollow">Urb Smart Device THCAP Disposable 6g</a>
<ahref="https://vapestorecart.com/product/vape-pen-and-charger-with-cbd-cartridge/" rel="dofollow">Vape Pen and Charger with CBD Cartridge</a>
<ahref="https://vapestorecart.com/product/cbd-vape-pen-cartridges/" rel="dofollow">CBD Vape Pen Cartridges</a>
<ahref="https://vapestorecart.com/product/jet-fuel-1000mg/" rel="dofollow">Jet Fuel [1000mg]</a>
<ahref="https://vapestorecart.com/product/thc-flowers/" rel="dofollow">THC FLOWERS</a>
https://cmminer.com/
https://cmminer.com/teach
https://cmminer.com/login?redirect=/campaigns
https://cmminer.com/about
https://cmminer.com/referral
https://cmminer.com/guide
https://cmminer.com/blog
https://cmminer.com/faq
https://sneydmining.com/contract
https://sneydmining.com/about
https://sneydmining.com/faq
https://sneydmining.com/tutorial
https://sneydmining.com/app-download
https://sneydmining.com/blog
https://sneydmining.com/signup
https://sneydmining.com/signin
https://sneydmining.com/
https://growingauto.com/
https://sneyd-mining.com/
https://sneyd-mining.com/favicon.ico
https://sneydmining.com/favicon.ico
https://cpspai.com/
https://www.cpspai.com/
https://www.cpspai.com/campaigns
https://www.cpspai.com/about
https://www.cpspai.com/login
https://www.cpspai.com/register
https://cpspai.com/campaigns
https://cpspai.com/about
https://cpspai.com/login
https://cpspai.com/register
The ultimate guide to Busan's nightlife, providing fast and accurate information on clubs, karaoke, and massage parlors, plus real-time reviews. 부산비비기 (https://busanbibigi.site)
How to Manage Brain Health: Brain Revitalize With Daily Health Routine 뇌건강관리
Public Transport Discount Cards: The best choice to save money on transportation 대중교통할인카드
Laptop Bag Recommendation: Style and Protection at the same time 노트북가방추천
Men's Hair Style Recommendation: Charm Upgrade and Battery Care Tips 남성헤어스타일
Introductory Golf Tips: A Practical Guide for Beginners and a Used Car Door Parable 골프입문팁
Air Purifier Recommendation: Best Choice and Shopping Mall Startup Tips for Pleasant Space 공기청정기추천
Marriage Checklist: Step-by-step requirements 결혼준비체크리스트
Health-style balance: Men's clothes that fit for men's clothes 건강식레시피
Health Checkup Items: Taking care of your health with essential tests and customized tips 건강검진팁
Romantic Autumn Train Trip: A Romantic Journey With Autumn Leaves 가을여행지추천
Smartwatch must-have: workout tracking and heart rate measurement perfect guide 가성비스마트워치
SNS Marketing Strategies: A Practical Guide to Brand Growth SNS마케팅
40s Health Care: Essential Habits to Overcoming Body Change 40대건강관리
Introductory Baseball Tips: Key Points For Beginners To Know 야구입문팁
1 person living tips: Digital currency forecast for digital currency forecast 1인가구생활
https://cpspai.com/
https://www.cpspai.com/
https://www.cpspai.com/campaigns
https://www.cpspai.com/about
https://www.cpspai.com/login
https://www.cpspai.com/register
https://cpspai.com/campaigns
https://cpspai.com/about
https://cpspai.com/login
https://cpspai.com/register
https://biolinky.co/credminer
https://blog.udn.com/G_115022164765959109/183332206
https://blog.udn.com/G_113368013016489159/183332203
https://blog.udn.com/G_104211651015257935/183332195
https://blog.udn.com/G_104723892582894659/183332193
https://blog.udn.com/G_104647200564078610/183332136
https://blog.udn.com/G_115022164765959109/183333386
https://blog.udn.com/G_113368013016489159/183333380
https://blog.udn.com/G_104211651015257935/183333376
https://blog.udn.com/G_104723892582894659/183333374
https://blog.udn.com/G_104647200564078610/183333372
https://planmining.com
https://planmining.com/home.html
https://planmining.com/login.html
https://planmining.com/register.html
https://planmining.com/about.html
https://planmining.com/app.html
https://planmining.com/FAQ.html
Rest-tel site through massage 휴게텔 사이트
OP Choices, Dalamsite Recommendations Make It Easier 달림사이트 순위 추천
Unlike other words, stable platform recommendations, stable platforms 달림사이트 순위 추천
https://goldenmining.cc/xml/index.html
https://goldenmining.cc/home.html
https://goldenmining.cc/blog.html
https://goldenmining.cc/app.html
https://goldenmining.cc/register.html
https://goldenmining.cc/login.html
https://goldenmining.cc/about.html
https://goldenmining.cc/
https://goldenmining.cc/xml/index.html
https://goldenmining.cc/home.html
https://goldenmining.cc/blog.html
https://goldenmining.cc/app.html
https://goldenmining.cc/register.html
https://goldenmining.cc/login.html
https://goldenmining.cc/about.html
https://goldenmining.cc/
https://planmining.com
https://planmining.com/login.html
https://planmining.com/register.html
https://planmining.com/about.html
https://planmining.com/app.html
https://planmining.com/FAQ.html
https://planmining.com/xml/favicon.ico
https://planmining.com/
https://cpspai.com/
https://www.cpspai.com
https://www.cpspai.com/campaigns
https://www.cpspai.com/about
https://www.cpspai.com/login
https://www.cpspai.com/register
https://cpspai.com/campaigns
https://cpspai.com/about
https://cpspai.com/login
https://cpspai.com/register
https://credminer.com/
https://credminer.com/contract
https://credminer.com/about
https://credminer.com/share
https://credminer.com/faq
https://credminer.com/tutorial
https://credminer.com/app-download
https://credminer.com/blog
https://credminer.com/signup
https://credminer.com/signin
https://goldenmining.cc/xml/index.html
https://goldenmining.cc/home.html
https://goldenmining.cc/blog.html
https://goldenmining.cc/app.html
https://goldenmining.cc/register.html
https://goldenmining.cc/login.html
https://goldenmining.cc/about.html
https://goldenmining.cc/
https://fleetmining.com/
https://fleetmining.com/pages/user/mine
https://fleetmining.com/pages/item/view
https://fleetmining.com/pages/hunter/view
https://fleetmining.com/pages/vip/index
https://fleetmining.com/pages/tutorial/view
https://fleetmining.com/pages/faq/view
https://fleetmining.com/pages/about/view
https://www.xiushanmining.com
https://xiushanmining.com
https://xiushanmining.com/home.html
https://xiushanmining.com/login.html
https://xiushanmining.com/register.html
https://xiushanmining.com/about.html
https://xiushanmining.com/app.html
https://xiushanmining.com/blog.html
https://profitablemining.com/index
https://profitablemining.com/contracts
https://profitablemining.com/affiliates
https://profitablemining.com/aboutus
https://profitablemining.com/vip
https://profitablemining.com/faq
https://profitablemining.com/tutorial
https://profitablemining.com/app
https://profitablemining.com
https://profitablemining.com/favicon.ico
Check here to find the Officetel address! 오피스타(https://getopstarturl.online) .
To learn about Sejong's 'hugyeteol,' come here to https://sejonghtel.com 세종시 휴게텔.
혼자 가는 서울 카페 여행, 남자의 여유 https://manincafeseoul.com.
Ulsan OP Perfect Guide: Tips and Information for Smart Choices 울산 오피
Cheongju Restel: A Special Space for Rest and Recharge 청주 휴게텔
Ansan OP: The choice for the perfect private break 안산 오피
The vibrant city of Daegu returns busily from day to night. In the meantime, there are times when you pause and desperately want a completely personal break. 대구 휴게텔
Pyeongtaek OP - Perfect Privacy Found in Complex Cities 평택 오피
Seosan OP - Perfect Privacy Found in Complex Cities 서산 오피
Looking for truly reliable Daegu OP information in a sea of confusing information? Based on rigorous standards and the latest data, our platform provides the most accurate and practical information that will save you precious time and effort. 대구 오피
Busan OP
Don't wander anymore. Here's the perfect break you've been looking for.
In the ever-changing flood of information, it's not easy to find truly reliable choices. 부산 오피
In the arduous daily lives of modern people, true relaxation and perfect recharging are not just luxuries but essential elements. Gangnam OP understands these deep needs, and provides the best premium healing solution for you only in Gangnam's core area. 강남 오피
Struggling to find truly reliable Gunsan OP information in a sea of confusing information? Now waste no more time and energy. Our Gunsan OP will meet all your expectations by providing the best information proven with strict standards and unrivaled insights. 군산 오피
Our Yeosu OP goes beyond just providing services and wants to provide unforgettable experiences and deep satisfaction to each and every customer. 여수 오피
Leggings Room: Managers come into Choice as leggings. After receiving a guest's choice, during Greetings, they change their top into see-through mesh see-through tops. 강남레깅스룸
Your exhausting day in Daejeon, you've been wondering where to get real comfort and recharging. Now put down your worries about uncertain information and disappointing experiences. 대전 오피
"The Car Remote Start Repair Awards: The Most, Worst, And The Most Unlikely Things We've Seen car keyless start system repair
There were many cases where it was difficult to find a reliable, satisfactory OP in Gwangju, and there was a lot of information, but it was hard to trust, right? 광주 오피
How much time have you wasted trying to find a truly satisfying experience in Andong? To avoid getting lost amid uncertain information and unverified options 안동 오피
Amid a flood of information, finding truly satisfying premium services is not an easy task. If you face uncertainty, wasting time and energy, don't hesitate anymore. Iksan OP exists to provide the perfect experience you have dreamed of. 익산 오피
Amid the constant stress and fatigue, are you longing for a true rest? The Bucheon Restoration Hotel exists beyond just space to deeply understand your body and mind and provide the best healing. 부천 휴게텔
Have you ever wandered through numerous web pages to find reliable OP information in the Chungju area, and been anxious about uncertain information? 충주 오피
Ansan Resttel deeply understands this period of fatigue and provides a premium space to reinvigorate life beyond mere rest. 안산 휴게텔
This is the "ultimate haven" that will recharge your soul to perfection and fill your energy to move back into the world. Don't worry, I'll hold both your wallets and your satisfaction. 원주 휴게텔
Chuncheon Restell, a special secret base to reset your body and mind! Don't fight against the mirror with your tired face anymore. It's time to laugh! 춘천 휴게텔
Hong Dae-ki is a vital course of your soul, and brain is the essential course of modern people, and the brain is the essential course of modern people.This is your "Recharge zone" right now 홍대 오피
Our platform promises to provide transparent, accurate, and selected information to anyone looking for Seongnam OP information, thereby relieving fatigue in information search and serving as a criterion of trust to help make optimal choices. 성남 오피
https://hmining.com
https://hmining.com/xml/favicon.ico
https://hmining.com/login.html
https://hmining.com/register.html
https://hmining.com/app.html
https://hmining.com/product.html
https://hmining.com/about.html
https://hmining.com/affiliate.html
https://hmining.com/sitemap.xml
https://rmcmining.com
https://rmcmining.com/login.html
https://rmcmining.com/register.html
https://rmcmining.com/app.html
https://rmcmining.com/contract.html
https://rmcmining.com/about.html
https://rmcmining.com/affiliate.html
https://rmcmining.com/xml/favicon.ico
Looking for the perfect experience in Konkuk University? Amid the deluge of information, you may have been unsure which places would truly live up to your expectations and which choices would provide you with unrepentant satisfaction. 건대 오피
Finding a 'place' that truly meets your expectations amid tons of information can sometimes feel like a challenge. But that's it for you. Paju OP 파주 오피
https://futuromining.com
https://futuromining.com/sitemap.xml
https://futuromining.com/robots.txt
https://futuromining.com/favicon.ico
https://futuromining.com/home.html
https://futuromining.com/login.html
https://futuromining.com/register.html
https://futuromining.com/about.html
https://futuromining.com/app.html
https://mspminer.com/
https://mspminer.com/contract
https://mspminer.com/tutorial
https://mspminer.com/faq
https://mspminer.com/about
https://mspminer.com/datacenter
https://mspminer.com/welfare
https://mspminer.com/login
https://mspminer.com/register
https://mspminer.com/favicon.ico
https://mspminer.com/sitemap.xml
https://mspminer.com/robots.txt
https://ltccloudmining.com
https://ltccloudmining.com/home.html
https://ltccloudmining.com/login.html
https://ltccloudmining.com/register.html
https://ltccloudmining.com/about.html
https://ltccloudmining.com/app.html
https://ltccloudmining.com/news.html
https://ltccloudmining.com/xml/favicon.ico
https://ltccloudmining.com/sitemap.xml
https://dgmining.com/
https://dgmining.com/about.html
https://dgmining.com/blog.html
https://dgmining.com/app.html
https://dgmining.com/login.html
https://dgmining.com/register.html
https://fedmining.com
https://fedmining.com/home.html
https://fedmining.com/login.html
https://fedmining.com/register.html
https://fedmining.com/about.html
https://fedmining.com/app.html
https://fedmining.com/affiliate.html
https://fedmining.com/favicon.ico
https://fedmining.com/sitemap.xml
https://sjmine.com
https://sjmine.com/home.html
https://sjmine.com/login.html
https://sjmine.com/register.html
https://sjmine.com/app.html
https://sjmine.com/about.html
https://sjmine.com/favicon.ico
https://ratemining.com
https://ratemining.com/app.html
https://ratemining.com/about.html
https://ratemining.com/register.html
https://ratemining.com/blog.html
https://ratemining.com/login.html
https://ratemining.com//sitemap.html
https://familymining.com
https://familymining.com/APP.html
https://familymining.com/login.html
https://familymining.com/register.html
https://familymining.com/about.html
https://familymining.com/blog.html
https://familymining.com/sitemap.html
Sokcho's finest retreat: Luxurious comfort, serene healing awaits. https://sokchotel.com 속초 휴게텔.
Discover Jeju's ultimate luxury escape. Unwind in comfort, revitalize your soul. https://jejutel.com 제주 휴게텔.
Stressed in Daegu? Melt tension away with our premium dry massage for total relief. https://daegugeonma.online 대구 건마.
Seosan Men: Melt away stress & revive. Professional massage, flexible hours! https://seosanmassage.online 서산 마사지.
Discover Yongin's secret to unwinding. Exclusive care, perfect for your quiet escape. https://yongingm.online 용인 건마.
Dangjin's best-kept secret for stress relief. Private, professional massage. https://dangjingunma.online 당진 건마.
Cheongju's premier male massage: Professional hands for ultimate rejuvenation. https://cheongjugunma.online 청주 건마.
Your premium escape awaits! Namyangju OP guarantees satisfaction & privacy. https://namyangjuopi.com 남양주 오피.
Tired? Yeongdeungpo offers professional relaxation in a clean, cozy setting. https://ydpkiss.online 영등포 키스방.
Couples, find your bliss! Private, cozy Gumi dates for unforgettable memories. https://gumikiss.online 구미 키스방.
Experience luxury at home! Top mobile massage reviews you can trust. https://biztripreview.com 출장 마사지 후기.
Searching Yeoju officetels? Find prime locations, best deals & smart investments here! https://yeojuopi.com 여주 오피.
Room Salon 2차 decoded: transparent info for your perfect night out. https://leggsrcha.online 룸싸롱 2차.
Lost vitality in Gunsan? Personalized massage restores balance & energy. Experience it! https://gunsanmassage.online 군산 마사지.
Gimhae OP: Trusted info, real reviews. Compare services & choose your ideal relaxation. https://gimhaeop.com 김해 오피.
Your desires, exquisitely met. Experience ultimate bliss with expert massage. https://roomclub.online 마사지 대딸.
Mokpo Dry Massage: Melt away stress & fatigue. Find your peace here! https://mokpogm.online 목포 건마.
Need to unwind in Anyang? Experience discreet comfort and friendly encounters. https://anyangkiss.online 안양 키스방.
Authentic Room System: Discover the true standard of premium entertainment. https://gangnampub.online 정통룸 시스템.
Uncover Ho Chi Minh's secret nightlife. Rooftops, jazz, and hidden gems! https://hcmcnightlife.com 호치민 밤문화.
Your night, elevated. Unforgettable moments await at Gangnam Ten Cafe. https://karopubcha.online 강남 텐카페.
Hanam's #1 healing secret revealed: Top-tier private relaxation, perfectly accessible. Dive in! https://hanamop.com 하남 오피.
Tired in Gunsan? Rejuvenate your body and mind with expert massage tips. https://gunsangm.online 군산 건마.
Curious about "Cheongnyong"? Its true meaning will electrify your senses. https://shirtsroomtt.online 청룡 뜻.
Unlock Yeouido's best offices! Optimal locations & smart prices for you. https://yeouidoop.com 여의도 오피.
TenPro 2nd Round: Clear costs, no surprises. Your trusted guide! https://thetenpro.work 텐프로 2차.
Unlock HCMC's electrifying nightlife. Your complete guide starts here! https://hcmnights.com 호치민 밤.
Hanoi, HCMC, Da Nang: Your safe passport to Vietnam's vibrant nights. https://vietnamsnight.com 베트남 유흥.
Craving a unique night? Gunsan offers pure comfort, gentle touch, tailored just for you. https://bizroomga.online 군산 키스방.
Late night stress? Jeonju's top massage. Professional touch for deep relaxation. Click! https://jeonjumassage.com 전주 마사지.
Feeling drained? Namyangju's private massage offers tailored relaxation. https://nyjmassage.online 남양주 마사지.
Sillim's exclusive 1-on-1 massage for men. Ultimate relaxation awaits! https://sillimmasaji.online 신림 마사지.
Redefine luxury travel. Discover Vietnam's bespoke VIP Emperor Tour! https://vtnkingtour.com 베트남 황제투어.
Seongnam's ultimate escape: Pro dry massage reviews for deep relaxation. Unwind now! https://seongnamgm.online 성남 건마.
Hanoi Emperor Tour: Enjoy safe, trusted nightlife & transparent prices. Click! https://hanoifun.com 하노이 유흥.
Sadang men: Escape stress with premium Swedish massage. Rejuvenate now! https://sdgunma.online 사당 건마.
Discover Nonsan's secret to pure bliss. Tailored massage for ultimate comfort! https://nonsanmassage.online 논산 마사지.
Ochang Men: Melt fatigue! Discover premium massage & reclaim your vitality. https://ochanggm.online 오창 건마.
Pohang's 'Homerun' HQ: Your sweet escape from stress. Find bliss tonight! https://pohangkiss.online 포항 키스방.
Tired of Seoul? Find your tranquil, private escape at Sadang O-pi. Recharge now! https://sadangop.com 사당 오피.
Unleash your desires. Ultimate thrills & discreet luxury await. Explore now! https://seoulrooms.online 하드코어 업소.
Need true rest? Unwind completely at Poseung Hyugetel, your perfect escape. https://poseungtel.com 포승 휴게텔.
Customers give lip service to managers? Discover 'Yeok-lip's' true meaning! https://gnkaroicha.online 역립 뜻.
Unlock Gangnam's secret. Your perfect night, chosen beyond the mirror. https://gnroomsallon.online 강남 미러룸.
Unlock the 'Shirt Room' secret: Understand its system, costs & charm! https://jjeomo.online 셔츠룸 뜻.
Swing into Gangnam fun! Easy batting, friendly help. Your perfect night out. https://gangnamroom.online 강남 야구장.
Discover Ilsan's late-night secret for ultimate healing & comfort. https://ilsankb.online 일산 키스방.
Busy? Pyeongtaek's high-class massage awaits. Melt stress & find your vitality, anytime! https://pyeongtaekgunma.online 평택 건마.
Escape to Gwangju's exclusive romantic retreat. Craft your perfect memories! https://gwangjukiss.online 광주 키스방.
Escape to Dongtan's cozy haven! Melt away fatigue & find true relaxation. https://dtrelax.com 동탄 마사지.
Discover Dongtan's exclusive 1-person massage. Rejuvenate in total privacy! https://dongtangunma.online 동탄 건마.
No hidden fees! Experience honest, luxurious after-parties with peace of mind. https://gangnamyuh.online 룸살룸 2차 가격.
Osan men, stressed? Unwind completely at our premier dry massage sanctuary. https://osangm.online 오산 건마.
Byeongjeom Massage Review: Melt stress, soothe muscles. Find your ultimate escape here! https://bjmassage.online 병점 마사지.
Gwangju's ultimate Swedish massage? We found the verified experts for your bliss. https://gwangjuswedi.com 광주 스웨디시.
Unlock top entertainment secrets! Expert-curated reviews & verified venue info. Choose smart. https://funcomu.com 유흥 커뮤.
Sejong's ultimate escape for tired men. Professional care, deep relaxation. Rejuvenate! https://sejongsigm.online 세종시 건마.
Unwind & recharge in Yeoksam! Professional male massage. Clean, comforting, convenient. https://yeoksammasaji.online 역삼 마사지.
Experience premium Anyang Swedish massage without the premium price. Your guide! https://anyangswedish.com 안양 스웨디시.
Your exclusive pass to adult secrets. Unfiltered experiences await. https://obscenestory.com 난교 썰.
Yeosu men, unwind! Discover expert massage tips for deep rest, anytime. https://yeosumasaji.online 여수 마사지.
Leggings Room: Your special meeting starts with a warm smile. Feel instantly at ease, like visiting friends. https://hipublicga.online 레깅스룸 인사.
Tired, gentlemen? Hanam Massage brings deep relief, even late at night. https://hanammassage.online 하남 마사지.
Don't book blind! Uncover Dongtan's top Swedish shops with real reviews. https://dongtanswe.com 동탄 스웨디시.
Escape the city! Discover private Swedish massage havens in Magok. https://magokswedish.com 마곡 스웨디시.
Late-night relief in Yeongtong. Men's private massage for total healing. https://ytmassage.online 영통 마사지.
Planning a special Bangkok night? Discover insider Puying price secrets here! https://bkkpuyingpric.com 방콕 푸잉 가격.
Club prices a mystery? Unlock transparent rates & premium service, no hidden fees! https://yeonsinnaekiss.online 하이퍼블릭 주대.
Lost in US ETFs? ETF Lens cuts through the noise. Find your winning strategy today! https://usetflens.com ETF 렌즈 – 미국 ETF를 꿰뚫는 투자 인사이트.
Unlock ultimate relaxation in Changwon. Your private escape from fatigue awaits! https://changwongunma.online 창원 건마.
What's 'Hand Play'? Get the definitive, easy explanation here! https://roomsln.work 핸플 뜻.
Master business & pleasure in Gangnam. Unrivaled privacy & service. https://roomsallongga.online 강남 룸살롱.
Pattaya Walking Street prices: No rip-offs! Your ultimate bar guide. https://pattayawalk.com 파타야 워킹스트릿 가격.
Unwind after dark! Seomyeon's professional massage for men. Recharge your body & mind. https://seomyeonm.online 서면 마사지.
Discover Hongdae's hidden charm! Attractive managers, sweet moments. Your perfect escape awaits. https://hongdaekiss.online 홍대 키스방.
Yeoksam Full Salon: Drinks, songs, & ultimate fun, all in one perfect night. https://publicichaby.online 역삼 풀싸롱.
Overwhelmed? Find deep relaxation & energy at Jongno Massage. Open late! https://jongromassage.online 종로 마사지.
Tired? Sinchon's premium dry massage melts stress away. Relax now! https://sinchongm.online 신촌 건마.
Nha Trang entertainment costs? Find the full 'Cong Gai' price analysis here! https://kongkaitip.com 꽁까이 가격.
Experience Japan like never before: Gourmet, VIP, Emperor style! https://jpkingtour.com 일본 황제투어.
Discover Seosan's best Swedish massage for deep relaxation and healing. https://seosanmassage.com 서산 스웨디시.
Don't just pay. Invest in unforgettable luxury & timeless memories. https://missyroom.online 룸살룸 가격.
Yeosu men, unwind! Professional massage for ultimate relaxation & vitality. https://yeosugunma.online 여수 건마.
Stay ahead in SEO! Discover verified trends, tools & community from one essential link. https://linkghost.xyz SEO HOT LINK.
Chungju's best Swedish massage? Top picks, reviews & health benefits inside! https://chungjuthe.com 충주 스웨디시.
Experience the pinnacle of discreet stocking pleasure. Read our review. https://stockingsjob.com 스타킹 대딸.
Curious about 'Boom Boom'? Get the real review & find your ultimate SE Asia bliss! https://asiamsg.com 동남아 마사지.
Unbiased Shirts Room reviews. See true service quality & staff professionalism before you visit! https://roomtctt.online 셔츠룸 후기.
Wonju Men: Unwind late. Expert massage for total body & mind relief. https://wonjumassage.online 원주 마사지.
Suyu's ultimate men's retreat: Luxurious Swedish massage for total fatigue relief, open late. https://suyugm.online 수유 건마.
Full Salon: Worth the cost? Discover insider pricing & enjoy without regrets. https://roomddeok.online 풀싸롱 가격.
Poseung men: Unwind your fatigue! Professional massage, clean space, even after hours. https://poseongmasaji.online 포승 마사지.
Ultimate relaxation in Geoje: Professional massage, delivered to you! https://geojemassage.online 거제 마사지.
Escape to Jinju's private Swedish sanctuary. Your ultimate 'me-time' begins. https://jinjuswedish.com 진주 스웨디시.
Seeking Da Nang's fresh nightlife? The Red Swing promises an unforgettable time. Details & costs inside! https://swingdanang.com 빨간그네 다낭.
Escape the city bustle! Indulge in Ho Chi Minh's soothing massage secrets. https://hcmmasage.com 호치민 마사지 정리.
Unwind in Busan! Melt away stress with personalized massage & restore balance. https://busanbody.com 부산 마사지.
Discover Gangnam's ultimate. Unrivaled luxury curated for the discerning 1%. https://gnbammoonhwa.online 강남 일프로.
Uijeongbu Refreshment! Expert massage for men. Late hours, pure bliss. Fatigue ends. https://uijbtherapy.com 의정부 마사지.
Stressed in Sokcho? Discover your private healing oasis for men. Pure bliss! https://sokchomasaji.online 속초 마사지.
Late night stress? Cheongju massage delivers expert relief 24/7. Rejuvenate! https://cheongjumasage.com 청주 마사지.
Tired? Discover Sokcho's finest dry massage. Elite relaxation for men. https://sokchogm.online 속초 건마.
Unlock ultimate relaxation in Hyangnam. Elite dry massage for busy men. https://hyangnamgm.online 향남 건마.
Ingyedong's hidden oasis: Indulge in a luxurious Swedish massage. https://ingyedongsw.com 인계동 스웨디시.
Tired? Unwind in Gimhae! Professional massage to melt stress & revitalize. https://gimhaegm.online 김해 건마.
Bundang Swedish Guide: Discover premium shops, exclusive programs & oils. https://bundangsw.com 분당 스웨디시.
Mokpo Outcall Massage: Your personalized escape delivered. Experience pure relaxation. https://mokpomassage.online 목포 마사지.
Escape to Namyangju's ultimate private retreat. Experience true intimacy. https://nyjkiss.online 남양주 키스방.
Experience Gangnam's best! Stunning beauties, top-tier service. Unrivaled. https://yuhungchoochun.online 강남 하이킥.
Osan men: Ditch post-work stress! Experience refreshing, late-night massage. https://osanrelax.online 오산 마사지.
Anyang men, stressed? Unwind after work with our professional massage. https://anyangbody.online 안양 마사지.
Unlock Vietnam's best massage! Get expert tips on types, skills & hygiene. https://vietnammsg.com 베트남 마사지.
Ulsan Men: Unwind tonight! Premium dry massage melts stress away. https://ulsangm.online 울산 건마.
Seeking a new thrill in Sinrim? Explore exclusive experiences & charming company. https://sillimkiss.online 신림 키스방.
Bundang's Exclusive Oasis: Experience Private, Tailored Relaxation. https://bdgunma.online 분당 건마.
Unlock the Public System: Clear guidance, honest prices, max satisfaction! https://publics.work 퍼블릭 시스템.
Treated like royalty? Honest Emperor Tour reviews unveil your majestic journey! https://viptourreview.com 황제투어 후기.
Discover Gwangyang's top massage choices! Find your perfect aroma or Thai healing. https://gymassage.online 광양 마사지.
Stressed, Iksan men? Unwind & revive. Expert massage, convenient hours. https://iksanbody.com 익산 마사지.
Gwangju stress? Find your oasis! Enjoy tailored, clean 1-person shop massage. https://gwangjugunma.online 광주 건마.
Experience Clark's Emperor Tour: Luxury golf, vibrant nights, privacy guaranteed. https://clarkkingtour.com 클락 황제투어.
What is Vietnam Boom Boom? Uncover unique massage techniques & reviews. https://vietnambooom.com 베트남 붐붐.
Lights, music, expert touch. Club-style massage: your ultimate sensory escape. https://jjeomoichaga.online 클럽식 안마.
Don't waste your night! Get the TRUTH on nightlife spots. Honest reviews. https://funsreviews.com 유흥 후기.
Nha Trang nights calling! Discover exclusive luxury & revitalizing experiences. https://ntrnight.com 나트랑 밤문화.
Want Bangkok's ultimate nightlife? Explore Agogo: fun, ladies & unique thrills! https://bkkagogo.com 방콕 아고고.
End your night perfectly. Discover luxurious romance & an unforgettable escape. https://roomsalonghg.online 룸 2차.
Experience Ulsan's top Swedish therapy. Your true healing journey starts! https://ulsanswedish.com 울산 스웨디시.
Pattaya KTV prices revealed! Discover how to enjoy Pattaya's nightlife affordably. https://pattayaktv.com 파타야 ktv 가격.
Experience your night's peak! Hyperbolic 2nd delivers premium luxury. https://gnyuhungups.online 하이퍼블릭 2차.
Hongdae never sleeps! Discover your 24/7 Swedish massage oasis for ultimate calm. https://hongdaeswedi.com 홍대 스웨디시.
HCMC's electrifying nightlife! Your comprehensive guide to all the fun. https://nighthcm.com 베트남 호치민 유흥.
Unleash your inner star! Dalto Karaoke: sing freely, laugh loud, sweet nights. https://publickaraoke.online 달토 가라오케.
Unlock Pattaya's nightlife: Your ultimate, stress-free guide starts here! https://pattayaprice.com 파타야 유흥.
Car Remote Key Repair Tips To Relax Your Daily Lifethe One Car Remote Key Repair Trick That Every Person Should Learn Car Remote Key Repair
10 Things That Your Family Teach You About Car Key Housing Repair car key housing repair
Unlock bliss in Icheon! Your guide to tailored Swedish massage for ultimate relaxation. https://icheonswedi.com 이천 스웨디시.
The 10 Scariest Things About Keyless Remote Repair Keyless Remote Repair
See What Car Keyless Start System Repair Tricks The Celebs Are Utilizing Car Keyless Start System Repair
Guide To Car Key Sensor Repair: The Intermediate Guide For Car Key Sensor Repair car key sensor repair
HCMC's Artistic Nights: Experience true freedom & luxury beyond fun! https://hcmclub.com 호치민 유흥.
Discover the best luxury tour countries! Elevate your travel experience. https://vvviptour.com 황제투어 나라 추천.
What happened overnight in the US? Get your quick daily news summary now! https://usnightnews.com US Night News.
Unlock Gangnam's luxury nightlife. Invest in an unforgettable experience! https://roomsalong.online 룸살롱 가격.
Don't guess! Our Haeundae Premium Swedish review ensures your satisfaction. https://haeundaeswedi.com 해운대 스웨디시.
Guide To Push To Start Key Repair: The Intermediate Guide Towards Push To Start Key Repair Push To Start Key Repair
Struggling with SEO? Access top sites & tools to boost your rankings now! https://bestblogurl.xyz 좋은 주소 모음.
Need to unwind? Find your bliss at Ansan's premier 1-person Swedish massage. https://ansanswedish.com 안산 스웨디시.
Vietnam Longtime prices revealed! No hidden costs, just fair deals. https://vietlongprice.com 베트남 롱타임 가격.
Pohang's special therapy awaits! Experience the gentle Swedish massage locals love. https://pohangswedi.com 포항 스웨디시.
Nha Trang Nightlife A-Z: Transparent prices, premium service, ultimate male comfort. Discover! https://nightvnm.com 나트랑 유흥.
Nha Trang Emperor Tour: Banish stress! Experience royal treatment & complete rejuvenation. https://vipviettour.com 나트랑 황제투어.
Your ultimate guide to Phu Quoc nightlife. Find your perfect vibe! https://phuquocnight.com 푸꾸옥 밤문화.
Your secret Da Nang fantasy awaits. Red Swing prices & honest reviews. https://swingvietnam.com 다낭 빨간그네.
Bangkok Long Time: Stop guessing! Get the *exact* Puing prices now. https://bkklongprice.com 방콕 롱타임 가격.
Da Nang Eco-girl prices & exclusive info. Your personal concierge is ready! https://danangkeyman.com 다낭 키맨.
Your Vietnam biz trip guide: Success, efficiency & luxury massage. https://vietnamstrips.com 베트남 출장.
Seoul brunch for men: Hearty meals, great vibes. Your ultimate weekend guide! https://seoulbrunch.com 서울 브런치 맛집, 남자의 선택.
Your PC, everywhere! Experience seamless, secure remote access now. https://remotedesk.site 원격 데스크톱.
Unlock API's power! From basic definition to diverse use cases, explained clearly. https://apimeaning.site api 뜻.
"Yar" decoded! Understand the trendy Gen Z slang's meaning & use today. https://yarmeaning.site 야르 뜻.
Triangle area formulas got you lost? Master all methods, basic to advanced. Easy! https://trianglearea.site 삼각형 넓이 공식.
Unlock powerful poster design! Master visuals with trendy methods & templates. https://posterdesign.site 포스터 디자인.
Unlock 24/7 profit! Discover the smart, staff-free cafe revolution. https://unmancafe.store 무인 카페 창업.
Open HWP documents easily! Download your FREE, secure Hangeul Viewer now. https://koreanfreeinstall.site 한글 무료설치.
Unlock the incredible secrets of your female body. Empower your health journey! https://womensform.site 여자 몸.
Timo Werner: Explosive pace, key goals, & crucial transfer updates. Read now! https://timowerner.site 티모 베르너.
Stressed Seoul men: Discover the city's best male-friendly massages, hand-picked for you. https://seoulmanspa.com 서울 마사지 비교, 남자 기준.
Tired of boring hair? Discover 2024's top men's styles & find your perfect look! https://guyhairstyles.site 남자 헤어스타일.
Make your partner swoon! Discover irresistible cute lines for every occasion. Ignite their love! https://cutelines.site 애교 대사.
Unlock your wrist's potential! Men's smartwatch guide, zero confusion. https://smartmanwatch.com 남자 스마트워치 입문 가이드.
Diabetes symptoms demystified. From signs to prevention, empower your health. https://symptomcheck.site 당뇨 증상.
Planning Japan? Don't miss these vital essentials for a smooth journey! https://japantravelprep.site 일본 여행 준비물.
End cold sore discomfort! Get effective HSV-1 relief & prevention tips. https://herpesguide.site 헤르페스 1형.
Adventure awaits! Explore Korea's hidden gems, culture, and flavors. https://koreacorners.site 대한민국 구석구석.
Unleash your style! Find unique handmade gems & connect with makers. https://handmadewk.online 핸드메이드 위크.
Beyond scales & mirrors: SmartFit Life uses tech for personalized fitness success. https://smartfitlife.site 스마트핏 라이프.
Unsure of your Windows? Verify your OS & get crucial compatibility data fast! https://winversion.site 윈도우 버전 확인.
Stop guessing! Get scientifically proven dog advice for a happier life. https://wonbests.com 댕댕이 연구소.
Co-op, PvP, casual! Your ultimate 2-player game guide is here. https://duogames.site 2인용 게임.
PHEV puzzle solved! Compare models, boost efficiency, claim subsidies. https://phevguide.site 플러그인 하이브리드 자동차.
Transform your summer look! Men's hat styles for sun, shade & swagger. https://mensummercap.com 여름 남자 모자 스타일링 블로그.
Want an eco-friendly life? Discover easy, practical daily green tips! https://ecohabit.online 친환경 생활연구.
Unlock webtoon success! Master story, submission & serialization secrets. https://webtoonstart.site 웹툰 작가되기노트.
Hall of Mirrors, grand gardens: Uncover Versailles' artistic masterpieces. https://versaillespalace.site 베르사유 궁전.
Master REST API from concept to code. Build robust web services, level up your dev! https://restfulapi.site rest api.
Car questions? Get all your answers: news, reviews, tips, & more! https://iknowuno.com 카뉴스 톡(CarNews Talk).
Your A1 size guide: Get all specs, tips & perfect solutions for printing. https://aonesize.site a1 사이즈.
Your perfect wedding journey starts here! Grab the ultimate planning guide. https://weddinglist.space 결혼 준비 체크리스트.
Unlock your perfect spring! ???? Get 2024 Korea cherry blossom dates & festival info here. https://sakuraseason.site 벚꽃 개화 시기.
Crypto investment: Is your money safe? Uncover hidden risks & fraud now! https://coinwatch.online 코인투자 주의보.
Stand out! Craft a developer portfolio that truly gets you hired. https://mydevpath.site 개발자 포트폴리오.
Searching for the perfect look? Find stylish people illustrations to bring your vision alive! https://peopleillus.site 사람 일러스트.
Your daily dose of joy! Discover heartwarming animal moments in stunning HD. https://adorableanimals.site 귀여운 동물.
Stop guessing 'chill'! Uncover its diverse meanings & confidently use it now. https://chillmeaning.site chill 뜻.
Master your PC's specs! Get pro insights for upgrades & gaming. https://pcspecs.site 컴퓨터 사양 확인.
Unlock SME success! Simplify policies, find grants, & fuel your business growth. https://smepolicy.space 중소기업 정책통.
Ride to fitness at home! Expert tips & reviews for your perfect indoor bike. https://homebiker.com 실내 자전거 추천 및 사용기.
No time for the gym? 30s men's home workouts for a sculpted body. Start now! https://manhomefit.com 30대 남자 홈트 루틴 정리 블로그.
Turn your YouTube hobby into steady income. Master monetization strategies! https://ytrevenue.space 유튜브 수익가이드.
"Unji" decoded: The unbiased truth behind its origin & controversies. https://fingerterm.site 운지 뜻.
Transform your look! Find your perfect Men's Hippie Perm & styling tips. https://manhippieperm.site 남자 히피펌.
Dreaming of a smartwatch? Get essential features & save big. Your guide starts here! https://smartdealwatch.site 가성비 스마트워치.
Taste YOUR perfect brew! Homebrew Notes guides your unique beer journey. https://homebrewlog.site 홈브루 노트.
Stocks made easy! Learn investing basics in just 5 minutes a day. https://howknow.info 하루 5분 주식 맛보기.
Tired of complex economics? Get simple, actionable tips to make your money grow! https://knowabc.info 경제 쏙쏙 돈 되는 이야기.
Escape the heat! Easy install, powerful cooling, energy-efficient window ACs. https://windowac.site 창문형 에어컨.
Ride smarter, save bigger! Unlock top public transport card discounts. https://transitdeal.site 대중교통 할인 카드.
Laugh out loud! The ultimate cat meme encyclopedia awaits. Daily fun guaranteed! https://catmemes.site 고양이 밈.
Unlock tasty, budget-friendly office lunches! Simple recipes for men. https://manlunchbox.com 직장인 남자 도시락, 쉽고 맛있게.
Make them say YES! Craft a perfect interview intro that gets you hired. https://selfintro.site 면접 자기소개.
Daily quizzes for a sharper mind! Learn new facts and have fun every day. https://quizsense.site 상식퀴즈 사이트.
Digital detox? Permanently delete or deactivate Twitter with our easy guide. https://goodbyesocial.site 트위터 계정 삭제.
Building a cafe? Learn to slash costs: interior, equipment & more. Budget smarter! https://cafebudget.space 카페창업 예산법.
Confused by health trends? Get evidence-backed clarity for a healthier you. https://cpurams.com 헬스 인사이트 (Health Insight).
Unlock your phone's ultimate security! Safeguard your privacy easily. https://phoneguard.online 폰보안 가이드.
Struggling for the best PC? Unlock max performance for work/gaming, affordably! https://pcquotes.site 컴퓨터 견적.
Is your CPU secretly killing your PC? Discover optimal temps & fixes! https://cputemp.site cpu 온도.
Choosing a cat? Compare breeds effortlessly & find your ideal companion! https://catbreedhub.site 고양이 종류.
Unwind! Discover Seoul's most enchanting night walks & dazzling views. https://nightwalkseoul.com 서울 야경 산책로 모음.
A-Flu Guide: Symptoms, prevention, & quick recovery. Stay safe this winter! https://fluainfo.site a형 독감.
Unlock winning 1-min intro secrets. Impress interviewers effortlessly! https://introminute.site 1분 자기소개.
Stop guessing! Calculate your precise take-home pay after taxes & insurance. Get clarity. https://netsalary.site 연봉 실수령액.
Stop the search! Find YOUR ideal, personalized insurance with ease. https://insurepick.online 보험 비교 사이트.
2024 GPU Ranking: Top gaming performance & best value revealed! Find your perfect graphics card here. https://gpurank.site 그래픽카드 순위.
Boost your brainpower! Practical tips to stay sharp & vibrant for life. https://braincare.space 뇌 건강 관리법.
Just 5 minutes before work can transform your day. Discover how! https://preworkstretch.com 출근 전 스트레칭 루틴 정리.
Unleash cuteness! Dive into HQ Zanmang Loopy memes for every mood. https://luffymemes.site 잔망루피 짤.
Confused by real estate? Get your clear investment map & profit! https://investmap.site 투자맵 하우스.
First used car? Conquer fear! Your guide to smart, safe, confident buying. https://usedcar101.site 중고차 입문가이드.
Demystify AI! Get clear insights on its impact on your life & future. Prepare wisely. https://aiviews.online 인공지능 인사이트.
Elevate your typing! Premium mechanical keyboards, switches & designs. https://mechkeys.site 기계식 키보드.
Your ultimate guide to pickup trucks: Models, specs & smart buying tips! https://pickuptrucks.site 픽업 트럭.
Uehara Takako: All you need! Updates, profile, rare videos & exclusive content. https://takakouehara.site 우에하라 타카코.
Make every day better! Transform your home with easy, step-by-step decor plans. https://homeplan.space 집꾸미기 플랜북.
Tired of guessing? Our expert mouse guide ensures a no-regret choice! https://mousepicks.site 마우스 추천.
Boost your health naturally! Discover how Propolis strengthens immunity & fights inflammation. https://propolisbenefits.site 프로폴리스 효능.
Tired of endless scrolling? Discover your next perfect movie, tailored just for you! https://filmpicks.site 영화 추천.
Launch your online store, simplified! Turn your passion into profit today. https://shopbizguide.site 쇼핑몰 창업백서.
Craving calm? Unwind under stars! Best solo night fishing spots near Seoul revealed. https://nightfishman.com 밤낚시 혼자 가는 서울 근교.
Stop searching! Get easy screen capture shortcuts for Windows, Mac & Mobile. https://screenshortcut.site 화면 캡쳐 단축키.
Tired of dull commutes? Dive into captivating books chosen for men's rides! https://trainreads.com 전철에서 읽기 좋은 남자책.
Snoop Instagram anonymously! View profiles, stories, posts without logging in. https://socialspy.site 인스타 로그인 없이.
Flu or cold? Don't guess! Learn distinct symptoms & prevention tips today. https://flusymptoms.site 독감 증상.
Cook smart, eat well! Delicious & healthy air fryer recipes made easy. https://aircook.site 에어쿡 레시피.
Ready for your next adventure? Find hiking trails for every level, from scenic strolls to epic challenges. Explore now! https://hikemap.site 등산 코스 추천.
Design your ultimate male homebody haven. Unwind, game, and relax in style! https://homenman.com 집순이 말고 집돌이 인테리어.
Unlock your unemployment benefits! Easy guide to eligibility & payout. https://joblesshelp.site 실업급여 조건.
Tired of YouTube ads? Unlock ad-free viewing & enjoy seamless content! https://youtubeadfree.site 유튜브 광고차단.
Dream of car camping? Unlock expert tips for a comfortable, unforgettable first adventure! https://chabaklog.space 차박 입문일기.
Tired of tired mornings? Unlock science-backed habits for deep, refreshing sleep! https://mansleeping.com 수면의 질 올리는 습관 정리.
Experience real Korea! Eat, explore & live like a local with our guide. https://uwantiknow.com 로컬 코리아.
Want to see dazzling beauty & trends? Dive into women's captivating style! https://stunningwomen.site 예쁜 여자.
Drive smart. Hybrids: unmatched fuel economy & eco-friendly tech. Explore models! https://hybridhub.site 하이브리드 차량.
Unlock Yeongtong's best Swedish massage secrets for deep relaxation & relief. https://yeongtongsw.com 영통 스웨디시.
Find your perfect Yatap massage! Honest reviews on quality & skill. https://yatamassage.com 야탑 마사지.
Discover Japan's culinary heart: Sushi, ramen, izakaya, & recipes to try! https://japaneats.site 일본 음식.
Men, reclaim your time! Find the perfect robot vacuum effortlessly. https://mancleancare.com 남자를 위한 청소 로봇 비교기.
What defines handsome? Explore features, styles & find your ultimate type! https://handsomemen.site 잘생긴 남자.
Stress out? Find your perfect Suyu 1-person Swedish healing oasis now. https://suyulove.com 수유 스웨디시.
Unlock Nonhyeon's secret to pure bliss! Premium Swedish massage guide. https://nonhyeonsw.com 논현 스웨디시.
Headset hunt over! Experts guide you to your perfect gaming, work, or music audio. https://headsetpicks.site 헤드셋 추천.
Hiccups got you down? Find quick folk & science-backed remedies here! https://stophiccups.site 딸꾹질 멈추는 법.
Yuseong's ultimate Swedish escape. Melt stress away & feel refreshed! https://yuseongswed.com 유성 스웨디시.
Stress? Recharge in Dongdaemun! Private Swedish massage for mind-body bliss. See reviews. https://dongdaemuns.com 동대문 스웨디시.
40s body acting up? Stop guessing! Get tailored health solutions today. https://health40s.site 40대 건강 관리.
Escape to bliss in Jeongja-dong! Discover curated spa havens for body & mind. https://jjdswedish.com 정자동 마사지.
Recharge your mind & body in Gunjae! Find your perfect Swedish healing escape. https://gundaespa.com 건대 스웨디시.
Escape the routine. Your ultimate guide to digital nomad freedom awaits! https://diginomad.space 디지털 노마드.
Transform instant rice into gourmet meals, fast! Delicious & diverse recipes for busy lives. https://instantcook.site 즉석밥 레시피북.
Ready for a change? Your simple guide to a sustainable fitness routine! https://fitnessrut.online 헬스루틴 가이드.
Escape to bliss! Jeju Yeon-dong Massage: your dream-like oasis for body & mind. https://jejuyondong.com 제주 연동 마사지.
Unlock Naseong-dong's best private Swedish massage secrets & relax! https://naseongsw.com 나성동 스웨디시.
Go beyond likes! Learn SNS marketing secrets to build a thriving brand. https://snsstrategy.online SNS 마케팅 전략.
Be a smart cat parent! Master essential care for your feline's optimal health. https://catcareguide.space 반려묘 건강 관리.
Tired of city traffic? Micro mobility redefines your urban journey. Ride smart, save time! https://micromove.store 마이크로 모빌리티.
Your private oasis in Yangju! Experience bespoke Swedish aroma massage. https://yangjutouch.com 양주 스웨디시.
Unlock peak male vitality! Smart supplement guide for lasting health. https://supp4man.com 남자를 위한 영양제 추천 리스트.
Play is learning! Discover practical ways to boost your child's cognitive & emotional growth. https://kidplayedu.store 유아놀이 교육법.
Unwind & rejuvenate! Discover Bongmyeong-dong's top-rated Swedish massage spas. https://bongmyeongsw.com 봉명동 스웨디시.
Jeongja-dong's top Swedish & Lomi Lomi spas: Your guide to ultimate bliss! https://jeongjadongsw.com 정자동 스웨디시.
Unlock Eunpyeong's top massages! Ranked shops for ultimate relaxation. https://eunpyeongsw.com 은평 스웨디시.
Michuhol-gu Swedish: Discover curated shops offering premium massage & great value. https://mchswedish.com 미추홀구 스웨디시.
Yullyang-dong's Best Private Swedish Massage? Discover Top-Rated 1-Person Shops! https://yulldongswed.com 율량동 스웨디시.
Sinchon Swedish: Tired & heavy? Detox, boost lymph, feel instantly lighter! https://sinchonsw.com 신촌 스웨디시.
Indulge in bliss! Gyodong's top massage. Rejuvenate your body & soul. https://gangneungmsg.com 강릉 교동 마사지.
Unwind in Dongtan! Discover premium Swedish massage for deep relaxation. https://dongtansw.com 동탄 북광장 스웨디시.
Unwind in Gwangju Sangmu-jigu! Find your perfect Swedish massage sanctuary. https://sangmusw.com 상무지구 스웨디시.
Discover Nampo-dong's finest Swedish. Unwind with sensory bliss! https://nampodongsw.com 남포동 스웨디시.
Songtan: Your private oasis. Personalized Swedish massage in a tranquil 1-person shop. https://songtanswedish.com 송탄 스웨디시.
Samsan-dong Swedish Massage: Your curated guide to blissful, affordable relaxation! https://samsanswedish.com 삼산동 스웨디시.
Boost your health! Gimhae Swedish reveals lymph flow secrets. https://gimhaespa.com 김해 스웨디시.
Dujeong-dong Massage: Unbiased reviews unveil top spots. Make a smart choice! https://dujeongthe.com 두정동 마사지.
Andong Okdong's best massage? Find your perfect spa with trusted reviews! https://andongmass.com 안동 옥동 마사지.
Yangjae's best Swedish massage shops, hand-picked for your ultimate relaxation. https://yangjaeswed.com 양재 스웨디시.
Unlock Gumi Indong's best massages! Honest reviews, prices & facilities. https://gumiindong.com 구미 인동 마사지.
Discover Gangbuk's top Swedish massage spots! Unwind & rejuvenate. https://gangbukrelax.com 강북 스웨디시.
Yatap's secret to bliss! Find your ultimate private Swedish massage sanctuary. https://yatopsg.com 야탑 스웨디시.
Seeking Yeouido's best Swedish massage? Get expert tips & top recommendations here! https://yeouidorelax.com 여의도 스웨디시.
Mokpo Pyeonghwa Plaza's Top Massage Shops! Find your perfect healing spot. https://mokpomass.com 목포 평화광장 마사지.
Escape the daily grind! Suwan District Swedish massage for total rejuvenation. https://suwanjisw.com 수완지구 스웨디시.
Lost finding the best Sindorim massage? Uncover verified Swedish spa reviews here! https://sindorimsw.com 신도림 스웨디시.
Bulldang-dong Swedish guide: We've analyzed the best shops for your peace of mind. https://buldangswed.com 불당동 스웨디시.
Hwaseong Swedish: Top shops ranked by reviews! Find your ultimate relaxation. https://hwaseongsw.com 화성 스웨디시.
Escape to Hanam! Experience profound healing at top 1-person Swedish shops. https://hanamsens.com 하남 스웨디시.
Your perfect Cheonho private Swedish spa awaits! Find transparent prices here. https://cheonhoswed.com 천호 스웨디시.
Ultimate relaxation in Yuseong! Discover trusted spas with user reviews & rankings. https://yuseongmass.com 유성 마사지.
Pure bliss awaits! Experience Gyodae's top-rated Swedish massage, guaranteed. https://gyodaeswed.com 교대 스웨디시.
Unlock ultimate relaxation in Gimhae Naewe-dong! Top-ranked massage & spa picks. https://gimhaemass.com 김해 내외동 마사지.
Unwind in Gumi Indong! Personalized Swedish & Lomi Lomi for deep emotional calm. https://gumiindongsw.com 구미 인동 스웨디시.
Silence the city. Experience serene Swedish relaxation in Changdong. https://changdongsw.com 창동 스웨디시.
Discover Hyunpung's secret to serenity: bespoke Swedish massage, just for you. https://hyeonpung.com 현풍 스웨디시.
Don't guess! Get the best Naseong-dong massage with trusted recommendations. https://naseongspa.com 나성동 마사지.
Need to unwind? Find your perfect Cheongdam private Swedish massage. https://cheongdamsw.com 청담 스웨디시.
Tired of searching? Discover Anseong's best Swedish massage, guaranteed spotless! https://anseongtouch.com 안성 스웨디시.
Sokcho's Sea & Swedish Bliss: Your ultimate healing escape awaits! https://sokchorelax.com 속초 스웨디시.
Byeongjeom Swedish: Detox, glow, and feel amazing! Your best massage awaits. https://bjswedish.com 병점 스웨디시.
Tired of searching? Discover Sangnam-dong's top private massage shops & tips. https://sangnammass.com 상남동 마사지.
Siheung Swedish: Unlock top-rated spots for ultimate healing & value! https://siheungswed.com 시흥 스웨디시.
Wirye Swedish: Experience a warm embrace. Discover top-tier relaxation today. https://wiryerelax.com 위례 스웨디시.
Songpa Swedish Massage Guide: Top spots & honest reviews revealed! https://songpaspa.com 송파 스웨디시.
Awaken your senses & soul! My La Festa Swedish review reveals the ultimate escape you deserve. https://lafestasw.com 라페스타 스웨디시.
Kyungsung Swedish: Don't guess! User-approved shops for guaranteed bliss. https://gyeongdaesw.com 경성대 스웨디시.
Stressful day? Unwind completely! Yangyang Swedish massage secrets here. https://yangyangsw.com 양양 스웨디시.
Seeking deep relaxation? Discover Hyangnam's top Swedish massage experience. https://hyangnamlove.com 향남 스웨디시.
Seolleung: Your private Swedish retreat. Escape the world, find your peace. https://seolleung.com 선릉 스웨디시.
Guide To Car Remote Start Repair: The Intermediate Guide Towards Car Remote Start Repair car remote start repair
What's The Current Job Market For Car Keyless Entry System Repair Professionals Like? Car Keyless entry system repair
The 9 Things Your Parents Taught You About Car Key Remote Repair Car Key Remote Repair
See What Car Key Blade Repair Tricks The Celebs Are Using Car Key Blade Repair
The Reason Behind Ignition Key Repair Is The Most Sought-After Topic In 2025 Keyless entry repair
Car Key Repair Explained In Less Than 140 Characters Emergency Car Key Repair
What's The Job Market For Car Keyless Entry System Repair Professionals Like? Car Keyless Entry System Repair
20 Things You Should Be Educated About Car Key Repair Service Remote Key Repair
5 Killer Quora Answers To Keyless Entry Remote Repair keyless entry remote repair
Smart Key Repair Tools To Streamline Your Daily Lifethe One Smart Key Repair Trick That Every Person Must Know Smart Key Repair
The 10 Most Terrifying Things About Affordable Car Key Repair Car Key Repair (Mclean-Lindgreen-4.Thoughtlanes.Net)
Why Keyless Entry Repair Will Be Your Next Big Obsession vehicle keyless entry repair (https://codimd.fiksel.info/bbxqq5wos8m5tuesy9mjpa/)
Car Remote Key Repair Tools To Make Your Daily Life Car Remote Key Repair Technique Every Person Needs To Learn car remote key repair (https://www.mixcloud.com/)
13 Things About Remote Key Repair You May Never Have Known Broken Key Repair
What's The Current Job Market For Car Keyless Entry System Repair Professionals? Car Keyless Entry System Repair
Five Killer Quora Answers On Keyless Entry Remote Repair Keyless Entry Remote Repair
15 Startling Facts About Smart Key Repair You've Never Known car Smart key repair
Guide To Car Remote Start Repair: The Intermediate Guide On Car Remote Start Repair Car Remote Start Repair
You'll Be Unable To Guess Flip Key Repair's Secrets Flip Key Repair
What's The Current Job Market For Keyless Fob Repair Professionals Like? Keyless Fob Repair
It's actually very difficult in this busy life to listen news on TV, therefore I only use world wide web for that purpose, and take the newest news.
The 9 Things Your Parents Teach You About Car Flip Key Repair Flip Key Repair (Https://schwanger.Mamaundbaby.com)
The 10 Most Terrifying Things About Laser Cut Key Repair Laser Cut Key Repair
The Reason Everyone Is Talking About Keyless Entry Repair This Moment Vehicle Keyless Entry Repair (https://notes.Io/WWiyN)
Car Key Shell Repair Tools To Help You Manage Your Everyday Lifethe Only Car Key Shell Repair Trick That Every Person Must Learn Car Key Shell Repair
10 Things Everybody Hates About Keyless Remote Repair Emergency Key Repair (Https://rentry.Co/xy8Of569)
I like it when folks get together and share opinions. Great site, stick with it!
Car Remote Key Repair Tools To Ease Your Everyday Lifethe Only Car Remote Key Repair Trick That Everyone Should Know Car Remote Key Repair
Car Remote Key Repair Tools To Ease Your Everyday Lifethe Only Car Remote Key Repair Trick Every Person Should Know Car Remote Key Repair
10 Things That Your Family Taught You About Vehicle Key Repair Vehicle Key Repair
Are You Responsible For A Car Mechanical Key Repair Budget? 12 Top Notch Ways To Spend Your Money Car Keyless Unlock Repair (Https://Fournier-Gupta-4.Technetbloggers.De)
Car Key Shell Repair Techniques To Simplify Your Daily Lifethe One Car Key Shell Repair Trick That Everybody Should Know car Key shell repair
Guide To Car Keyless Entry Fob Repair: The Intermediate Guide On Car Keyless Entry Fob Repair Car Keyless Entry Fob Repair
See What Keyless Entry Repair Tricks The Celebs Are Using keyless entry repair (https://rentry.co/)
This article will help the internet viewers for building up new blog or even a weblog from start to end.
5 Killer Quora Answers On Car Mechanical Key Repair Car Mechanical Key Repair
The 10 Most Terrifying Things About Keyless Entry Remote Repair Keyless Entry Remote Repair
Nine Things That Your Parent Taught You About Vehicle Key Fob Repair Vehicle Key Fob Repair
See What Emergency Key Repair Tricks The Celebs Are Using Emergency Key Repair
These are genuinely impressive ideas in on the topic of blogging. You have touched some pleasant things here. Any way keep up wrinting.
It's very easy to find out any matter on net as compared to textbooks, as I found this piece of writing at this web site.
Ahaa, its good dialogue about this post at this place at this website, I have read all that, so at this time me also commenting at this place.
I will immediately take hold of your rss feed as I can’t to find your e-mail subscription link or e-newsletter service. Do you’ve any? Kindly allow me recognise in order that I may just subscribe. Thanks.
https://567seo.com
https://familymining.com/
https://ratemining.com/
https://fedmining.com/home.html
https://fedmining.com/login.html
https://ltccloudmining.net/
https://ltccloudmining.net/login.html
https://ltccloudmining.net/app.html
https://ltccloudmining.net/about.html
https://deleioncapital.com
https://deleioncapital.com/auth/login
https://deleioncapital.com/about
https://deleioncapital.com/auth/Signup
https://deleioncapital.com/support
https://wpahash.com/home.html
https://wpahash.com/login.html
https://wpahash.com/register.html
https://wpahash.com/about.html
https://wpahash.com/app.html
https://wpahash.com/contract.html
https://wpahash.com/news.html
I am sure this article has touched all the internet users, its really really nice post on building up new web site.
9 . What Your Parents Teach You About Emergency Car Key Repair Emergency Car Key Repair
9 Lessons Your Parents Teach You About Car Key Housing Repair Car Key Housing Repair
9 . What Your Parents Taught You About Car Key Housing Repair Car Key Housing Repair
Nine Things That Your Parent Teach You About Car Key Remote Repair Car Key Remote Repair; https://pad.geolab.space/A2Bk-fFsRr6fxkskrwk4jw/,
Here's A Few Facts About Car Key Repair Car Key Repair Service (mlx.su)
Guide To Car Lock Repair: The Intermediate Guide In Car Lock Repair car lock repair
10 Things Everybody Gets Wrong About The Word "Car Key Guard Repair" Key Fob Repair
I will right away grab your rss as I can not to find your e-mail subscription link or newsletter service. Do you have any? Please allow me recognize in order that I could subscribe. Thanks.
But wanna input on few general things, The website design is perfect, the written content is really great :D.
Car Key Shell Repair Tools To Ease Your Everyday Lifethe Only Car Key Shell Repair Technique Every Person Needs To Be Able To Car Key Shell Repair
Five Killer Quora Answers On Car Key Signal Issue Repair Car Key Signal Issue Repair
Nine Things That Your Parent Teach You About Best Robot Hoover Best Robot Hoover
9 Lessons Your Parents Teach You About Robot Hoover Uk Robot Hoover Uk
What's The Current Job Market For Autonomous Vacuum Professionals? Autonomous vacuum
See What Robot Vacuum Cleaners Uk Tricks The Celebs Are Using robot vacuum cleaners uk
Guide To Best Robot Vacuum Uk: The Intermediate Guide The Steps To Best Robot Vacuum Uk robot vacuum uk
8 Tips To Improve Your Robot Cleaners Uk Game buy robot Cleaner
What's The Job Market For Which Robot Vacuum Cleaner Professionals? which robot vacuum cleaner
15 Robot Vacuum And Cleaner Benefits Everyone Should Know smart dust cleaner
How Much Can Robot Vacuum Cleaners Uk Experts Earn? autonomous vacuum (www.kaseisyoji.com)
You'll Be Unable To Guess Robotic Vacuum Cleaners Uk's Benefits Robotic Vacuum Cleaners Uk; Www.Tomahawknation.Com,
Guide To Robot Vacuum Cleaner With Mop: The Intermediate Guide The Steps To Robot Vacuum Cleaner With Mop Robot Vacuum Cleaner With Mop
You'll Never Guess This Autonomous Vacuum's Benefits Autonomous Vacuum
The Reason Why You're Not Succeeding At Automatic Hoover Uk Robotic vacuum cleaner - pratt-buhl.hubstack.net -
15 Gifts For Your Robot Hoovers Lover In Your Life self-cleaning Vacuum
Robot Vacuum And Cleaner Tools To Help You Manage Your Daily Life Robot Vacuum And Cleaner Trick That Should Be Used By Everyone Be Able To robot Vacuum And Cleaner
Robot Cleaner Vacuum And Mop Tools To Streamline Your Daily Life Robot Cleaner Vacuum And Mop Trick Every Individual Should Know robot Cleaner vacuum and mop (skitterphoto.com)
Guide To Robot Vacuum Cleaner With Mop: The Intermediate Guide For Robot Vacuum Cleaner With Mop robot vacuum cleaner with mop
13 Things About Automatic Vacuum You May Not Have Considered automatic vacuum cleaners, https://schaefer-whitney-2.blogbright.net/Automatic-vacuum-cleaner-explained-in-fewer-than-140-characters-1756526666,
Ten Autonomous Vacuum That Will Change Your Life vacuum robot (https://pad.Karuka.tech)
https://monerominers.com/
https://monerominers.com/login.html
https://monerominers.com/register.html
https://monerominers.com/app.html
https://monerominers.com/about.html
https://monerominers.com/blog.html
https://etcmining.com
https://etcmining.com/home.html
https://etcmining.com/login.html
https://etcmining.com/register.html
https://etcmining.com/about.html
https://etcmining.com/app.html
https://etcmining.com/blog.html
https://etcmining.com
11 Ways To Destroy Your Robot Vacuum self-Navigating vacuum
See What Automatic Vacuum Cleaner Uk Tricks The Celebs Are Using automatic vacuum cleaner uk
See What Best Robotic Hoover Tricks The Celebs Are Using Best robotic hoover
20 Fun Facts About Robotic Hoover robotic hoover and mop (https://md.un-hack-bar.de)
Guide To Best Robot Vacuum Uk: The Intermediate Guide Towards Best Robot Vacuum Uk Best Robot vacuum uk - hso.moe -
Undisputed Proof You Need Robot Vacuum Cleaners robot cleaner (https://kastrup-neville-2.mdwrite.net/10-things-we-hate-about-robot-Hoover-and-mop-1756375716)
Guide To Robot Vacuum Cleaner With Mop: The Intermediate Guide Towards Robot Vacuum Cleaner With Mop robot vacuum cleaner with mop
5 Killer Quora Answers On Robot Vacuum Cleaner Uk robot vacuum cleaner uk
10 Quick Tips For Robovac Hoover robotic Vacuum Cleaner
Guide To Robot Vacuum Cleaner With Mop: The Intermediate Guide For Robot Vacuum Cleaner With Mop robot vacuum cleaner With mop
The Little Known Benefits Of Remote Hoovers self cleaning robot vacuum
The 10 Most Scariest Things About Floor Robot floor robot - https://torino.Com.Mx -
Guide To Auto Vacuum Cleaner: The Intermediate Guide The Steps To Auto Vacuum Cleaner Auto Vacuum
What's The Current Job Market For Best Automatic Vacuum Cleaner Professionals Like? Automatic vacuum cleaner
Automatic Vacuum: The Good, The Bad, And The Ugly automatic Vacuum cleaner
10 Things You've Learned In Preschool That Will Help You With Robot Vacuum Cleaner Robot Vacuum Cleaners Uk
You'll Never Guess This Flip Key Repair's Tricks Flip Key Repair (Https://School-Of-Safety-Russia.Ru/User/Kneelyric6/)
The 10 Most Terrifying Things About Floor Robot floor Robot
Why Keyless Entry Repair Is Everywhere This Year Vehicle Keyless Entry Repair (Pad.Fs.Lmu.De)
Guide To Automatic Hoover: The Intermediate Guide On Automatic Hoover Automatic Hoover
Five Tools That Everyone Who Works In The Mopping Robot Industry Should Be Using smart floor Cleaner
The 10 Most Terrifying Things About Best Robot Vacuums Uk best robot vacuums uk (vsegda-pomnim.com)
Automatic Vacuum Cleaner And Mop Tools To Streamline Your Everyday Lifethe Only Automatic Vacuum Cleaner And Mop Trick That Every Person Should Be Able To automatic vacuum cleaner (pad.geolab.space)
24-Hours To Improve Robotic Hoover Robotic Hoovers
Cheap Robot Hoover Tools To Ease Your Daily Life Cheap Robot Hoover Trick Every Individual Should Know cheap Robot hoover
5 Laws That Anyone Working In Robot Vacuum Cleaner Should Know best robot vacuum cleaners Uk
20 Myths About Auto Vacuum Cleaner: Busted best robotic vacuum Cleaner Uk
How To Beat Your Boss In Remote Vacuum Cleaner smart floor vacuum
What's The Job Market For Good Robot Vacuum Cleaner Professionals Like? good robot vacuum cleaner
Automatic Vacuum Cleaner And Mop Tools To Help You Manage Your Daily Lifethe One Automatic Vacuum Cleaner And Mop Trick That Everybody Should Know automatic Vacuum cleaner (xq.nyyxl.com)
As a devoted roulette enthusiast, I am perpetually enthralled by the hypnotic spin of the wheel. My blog is a deep dive into this iconic game of pure chance. I explore the differences between American, European, and French roulette and why it matters. We'll evaluate popular betting systems and the mathematical fallacies that often accompany them. My credo is that while you can't beat the odds, you can maximize your enjoyment and manage your bets intelligently. I narrate stories of incredible roulette wins and the history of this "Devil's Game." This is a celebration of roulette in all its simple, elegant glory. I am here to enhance your appreciation for the game and help you bet with a clear understanding. Let's place our bets and watch where the little white ball lands together.
https://huayutianchengplus.top:2300/magnoliaquigle
https://git.nussi.net/laraewittenoom
https://realtors.7venoaks.com/author/beatrizmobley6/
https://www.rhcapital.cl/employer/casinohub-365/
https://git.hsy.com/tarahmulkey638
http://git.365zuoye.com/sondrakie02236
https://crystalimmobilier.com/author/ferdinandmansf/
https://playtube.live//@carrollu712685?page=about
https://home-pitch.com/author/jessikadenker8/
https://volts.howto.co.ug/@judewynn374969
5 Laws That Can Help The Robot Vaccum Cleaner Industry robot Vacuum Cleaner
10 Things That Your Family Taught You About Robotic Vacuum Cleaner Uk robotic vacuum cleaner uk
"The Ultimate Cheat Sheet On Robot Vacuum Cleaner best robot vacuum cleaners uk (list.ly)
Smart Key Repair Tips To Relax Your Daily Life Smart Key Repair Trick That Everyone Should Know Smart Key Repair
15 Best Robot Vacuum Cleaners Bloggers You Need To Follow best robot vacuum cleaners
9 . What Your Parents Taught You About Robot Vacuum With Mop Best robotic vacuum cleaner uk
Cheap Robot Hoover Tips To Relax Your Everyday Lifethe Only Cheap Robot Hoover Trick That Should Be Used By Everyone Know Cheap robot Hoover
What's The Current Job Market For Which Robot Vacuum Cleaner Professionals Like? which Robot vacuum cleaner
The Most Common Mistakes People Do With Best Robot Vacuum And Mop Best Robot Vacuum And Mop Uk
Guide To Auto Vacuum Cleaner: The Intermediate Guide The Steps To Auto Vacuum Cleaner Auto Vacuum Cleaner
See What Robot Vacuum Cleaners Uk Tricks The Celebs Are Utilizing robot vacuum cleaners uk; xushui.ccshw.Cn,
The Biggest Issue With Auto Vacuum, And How To Fix It Self-charging Vacuum
Don't Make This Silly Mistake You're Using Your Automatic Hoovers Robotic Vacuum Device
The 10 Scariest Things About Robot Vacuums & Mops Robot vacuums & mops
7 Simple Tricks To Totally Doing The Floor Robot self cleaning robot vacuum
Cheap Robot Hoover Tools To Streamline Your Daily Life Cheap Robot Hoover Trick That Everyone Should Know Cheap robot Hoover
https://lookmining.com/
https://lookmining.com/home.html
https://lookmining.com/login.html
https://lookmining.com/register.html
https://lookmining.com/app.html
https://lookmining.com/about.html
https://lookmining.com/news.html
https://lookmining.com/FAQ.html
https://dcrmining.com/
https://dcrmining.com/login.html
https://dcrmining.com/register.html
https://dcrmining.com/app.html
https://dcrmining.com/about.html
https://dcrmining.com/blog.html
https://dcrmining.com/sitemap.xml
What's The Current Job Market For Best Automatic Vacuum Cleaner Professionals Like? best automatic vacuum cleaner
You said it perfectly.
See What Automatic Vacuum Cleaner Uk Tricks The Celebs Are Using Automatic Vacuum cleaner uk
The 10 Scariest Things About Floor Robot floor Robot
What's The Current Job Market For Best Automatic Vacuum Cleaner Professionals Like? Best Automatic vacuum Cleaner
See What Best Robotic Hoover Tricks The Celebs Are Making Use Of best robotic hoover
10 Best Robotic Hoover Tricks All Experts Recommend robotic Hoovers
You’re a master of the written word!
15 Secretly Funny People In Best Robot Vacuums Uk Robotic Cleaner
The 10 Scariest Things About Automatic Vacuum Cleaner automatic Vacuum Cleaner
The 10 Scariest Things About Best Robot Vacuums Uk best robot vacuums uk
20 Things Only The Most Devoted Buy Cleaning Robot Fans Should Know vacuum robot
Cheap Robot Hoover Tools To Ease Your Daily Lifethe One Cheap Robot Hoover Technique Every Person Needs To Learn cheap robot hoover
It's The Myths And Facts Behind Robot Vacuum Cleaners Uk best robotic Vacuum cleaner uk
Guide To Auto Vacuum Cleaner: The Intermediate Guide For Auto Vacuum Cleaner Auto Vacuum cleaner
Five Killer Quora Answers To Robot Vacuum Cleaner Uk robot vacuum cleaner uk
20 Things That Only The Most Devoted Buy Cleaning Robot Fans Should Know Robot Vacuum cleaners
Five Killer Quora Answers On Robot Vacuum Uk Robot Vacuum Uk
9 Lessons Your Parents Teach You About Automatic Vacuum Cleaners automatic vacuum Cleaner (Askmotopros.com)
5 Killer Quora Answers On Robotic Hoover Robotic Hoover
How Robot Vacuum Cleaners Uk Arose To Be The Top Trend On Social Media Autonomous vacuum
See What Best Robotic Hoover Tricks The Celebs Are Using best robotic hoover (http://spectr-sb116.ru/user/userain74)
How To Save Money On Robot Hoovers robot Vacuum
5 Killer Quora Answers To Robot Vacuum robot vacuum (copeland-Kumar.federatedjournals.com)
You'll Never Guess This Robot Mop Uk's Tricks robot mop uk (www.Ludikarus.com)
Blackjack is not a game of chance; it's a battle of wits, skill, and nerve. I am a devoted specialist, an author whose complete focus is on the art and science of blackjack. My blog is a comprehensive study into every facet of this classic game. I've devoted thousands of hours perfecting basic strategy and studying card counting systems. Let's journey far beyond just hitting or standing. I discuss everything from shuffle tracking to betting spreads and table selection. The aim is to give you the knowledge to actually beat the dealer. I am certain that with practice and dedication, you can turn the odds in your favor. It's time to own the game of 21 together.
https://nvuplayer.com/@ariel12608192?page=about
https://sparcle.cn/git/larue062399928
https://buildingraja.com/author/carmelawelsby/
https://www.pulaplay.com/@jensbeeston841?page=about
http://58.34.114.82:7998/jonathanmoreir/jonathan2004/issues/1
https://athensgrouprealestate.com/author/antonhenry2144/
https://goldlarimobiliaria.com.br/agent/elsabouldin253/
https://git.7vbc.com/jose9013495129
https://cozwo.com/read-blog/22831_a-balanced-view.html
http://wcollector.idatabank.com:5230/zenaidarosetta/8336374/wiki/Revolutionize+Your+%25CE%25B4%25CF%2589%25CF%2581%25CE%25B5%25CE%25AC%25CE%25BD+%25CE%25BA%25CE%25BF%25CF%2585%25CE%25BB%25CE%25BF%25CF%2587%25CE%25AD%25CF%2581%25CE%25B7%25CE%25B4%25CE%25B5%25CF%2582+Frumzi+With+These+Straightforward-peasy+Tips
What's The Current Job Market For Best Automatic Vacuum Cleaner Professionals Like? Best automatic Vacuum Cleaner
10 Things That Your Family Teach You About Robotic Vacuum Cleaner Uk Robotic vacuum cleaner uk
See What Best Robotic Hoover Tricks The Celebs Are Utilizing best robotic Hoover
What's The Job Market For Autonomous Vacuum Professionals Like? Autonomous vacuum
10 Robot Cleaner Uk-Related Projects To Stretch Your Creativity robotic vacuum
20 Fun Facts About Robotic Hoover robotic Hoovers
Fantastic data, Thanks a lot!
9 Lessons Your Parents Taught You About Robotic Vacuum Cleaner Uk robotic vacuum Cleaner uk
Guide To Auto Vacuum Cleaner: The Intermediate Guide To Auto Vacuum Cleaner auto Vacuum
Cheap Robot Hoover Tools To Make Your Daily Life Cheap Robot Hoover Trick That Every Person Should Be Able To Cheap Robot Hoover
You'll Never Be Able To Figure Out This Robotic Hoover's Tricks Robotic hoover
5 Killer Quora Answers To Robotic Hoover robotic hoovers
What's The Job Market For Good Robot Vacuum Cleaner Professionals? Good Robot Vacuum Cleaner
The 9 Things Your Parents Taught You About Robot Hoover Uk Robot Hoover uk
7 Simple Strategies To Completely Making A Statement With Your Vacuum Cleaners Robot self-cleaning robotic Vacuum
I'm extremely inspired with your writing skills as well as with the layout to your weblog. Is that this a paid theme or did you customize it your self? Anyway stay up the nice quality writing, it's rare to peer a great blog like this one today..
Good stuff. Cheers!
20 Insightful Quotes On Automatic Vacuum automatic vacuum Cleaners (www.Stampedeblue.com)
You'll Never Be Able To Figure Out This Robot Mop Uk's Tricks robot mop uk (md.swk-web.com)
See What Best Robotic Hoover Tricks The Celebs Are Using best robotic hoover
10 Things That Your Family Teach You About Auto Vacuum auto vacuum
I merge my enthusiasm for content creation with my deep knowledge of betting games. My real perspective and comprehensive reviews define my writing apart in the competitive gambling community.
http://africa2063.iambrandsdev.com/read-blog/15254_a-comunidade-do-cash-mania-game-jogue-com-colegas.html
https://git.daoyoucloud.com/daciaself62743/casino-officiel-ladbrokes1982/wiki/The-secret-Code-To-Sign-In-To-Ladbrokes.-Yours%2C-For-free...-Really
http://www.creatorengine.cn:8418/janethartnett5/alternativas-cash-mania1984/wiki/Explorando-as-Fun%C3%A7%C3%B5es-Sociais-do-Jogo-Cash-Mania
https://intunethings.com/inesstang28149
http://45.9.148.61:3000/dinoraine6017/obstawianie-w-bizzo2022/wiki/These-10-Hacks-Will-Make-You%28r%29-Bizzo-Casino-Zak%C5%82ady-Sportowe-%28Look%29-Like-A-pro
http://120.26.46.180:3000/kris9838995325/jogar-fortune-snake-sem-baixar2019/wiki/Crit%C3%A9rios-para-Selecionar-um-Excelente-Cassino-Online
https://samaj.aanwebsolutions.in/read-blog/17937_getting-the-best-software-program-to-power-up-your-coral-casino-rating.html
http://git.scdxtc.cn/garylumholtz45/hollywoodbets-code-promo5048/wiki/Hollywoodbets%3A-From-Durban-Roots-to-a-Global-Betting-Powerhouse
https://amfhomes.com/author/orvalmcquay18/
https://git.xuntakeji.com/hymancloutier/hyman1982/wiki/Why+Ignoring+Coral+Slots+Will+Cost+You+Sales
I prosper on the vigor of betting venues, routing that into my interesting articles. Readers profit from my truthful opinions on matches, rewards, and field news.
https://primeteamdeals.com/archives/author/georgianna61v
https://easydating.shop/@francescomoral
https://git.vicagroup.com.cn/angiekohler633
https://rejobbing.com/companies/jetx-777/
https://talentlinkjobs.co.uk/companies/fortune-rabbit-777/
https://kivutv.com/read-blog/3005_jammy-monkey-compared.html
https://gitlab.jmarinecloud.com/russncb2800425
https://museum.skeiron.com.ua/agent/marisa39493260/
http://bbhsoba.com.ng/read-blog/12586_the-thrill-of-aviator-soaring-high-with-risk-and-reward.html
https://voicync.com/bernadettedalu
9 . What Your Parents Teach You About Robot Hoover Uk Robot Hoover Uk (Md.Farafin.De)
Welcome to my personal exploration of the casino's soul. I'm an author who views the gaming floor as a living, breathing entity, a complex ecosystem of hope, strategy, and chance. My blog is a collection of essays and observations that delve into the philosophy and psychology behind our desire to gamble. I ponder questions like: why do we chase losses, and what makes a winning moment so euphoric? I analyze the architecture of casinos and how it's designed to influence our behavior. My writing is less of a "how-to" guide and more of a "why-we-do" reflection. I am fascinated by the intersection of risk, human nature, and game design. If you're looking for a more thoughtful, introspective take on the world of casinos, you have come to the right place. Let's contemplate the deeper meaning behind the games we play.
https://cvbankye.com/employer/alexandercasino-login-777/
https://code.luoxudong.com/garrettknight/8196402/wiki/Geldtransfer-im-1bet4win-Casino
https://codes.tools.asitavsen.com/kimberlymcgrif
http://47.76.126.2:3000/dawnamckeel356/4089golden-panda-casino/wiki/Golden+Panda+Casino%253A+A+Glimpse+into+a+World+of+Opulence+and+Opportunity
http://139.196.177.200/shawnaisaacs28/2563750/wiki/Esclarecendo-D%C3%BAvidas-Sobre-o-Jogo-Fortune-Rabbit
https://snapfyn.com/jamiescullin09
https://waslah.agency/employer/1bet-4win-casino/
http://47.76.126.2:3000/dawnamckeel356/4089golden-panda-casino/wiki/Golden+Panda+Casino%253A+A+Glimpse+into+a+World+of+Opulence+and+Opportunity
https://reeltalent.gr/employer/cash-mania/
http://47.109.37.87:3000/sterlingx6330
I'm a digital native who only plays at online casinos, and this blog reflects that singular focus. I've mastered the art of navigating the virtual casino world. My work is geared for the online player, exploring topics like choosing the best software, using e-wallets for fast payouts, and taking advantage of online-specific game variants. I do not talk about tipping dealers or finding a physical table. This is a pure, unadulterated guide to obtaining the best possible experience from your desktop or mobile device.
http://47.92.159.28/morachatfield/notes-alexander-casino1980/-/issues/1
https://www.workforce.beparian.com/employer/casinoalexander-777/
https://starzijproperties.ng/agent/isobelbarnum0/
http://106.55.61.128:3000/milagros82p935/8915973/wiki/4+Questions+Answered+About+Betfair+Betting+Site
http://pronorte.com.mx/agent/anitrakrier39/
http://106.55.61.128:3000/milagros82p935/8915973/wiki/4+Questions+Answered+About+Betfair+Betting+Site
https://mikropomoc.pl/profile/nolagillies415
https://nigeria-real-estate.com/author/berniegonyea46/
http://git.fast-fun.cn:92/rollandyhy756/coral-casino365.com9369/wiki/Four+Methods+You+can+Bet+At+Coral+Casino+Without+Investing+An+excessive+amount+of+Of+Your+Time
http://183.56.232.100:3062/latashia74k832/golden-panda-casino9771/wiki/Golden+Panda+Casino%253A+A+Glimpse+into+Opulence%252C+Entertainment%252C+and+Responsible+Gaming
I'm a gourmand and a spirits expert who believes that the contemporary casino resort experience is just as much about the food and bar options as it is about the gambling. The casino floor might be the main attraction, but the Michelin-starred eateries and high-end lounges are the soul. My blog is a one-of-a-kind fusion of a casino blog and a gastronomic travelogue site. I'll tell you where to get the tastiest buffet just feet from the poker room. I'll review the wine lists at the most popular resort lounges. Join me as we explore the total hedonistic delight that the leading gaming destinations have to provide.
http://naughtycat.biz:3333/auiterese19065
http://gitlab.digital-work.cn/pilarmaney024/5-gringo-sportwetten1254/issues/1
https://realty.livcre.com/author/linwaller99515/
https://firmarealestate.com/agents/loviegerman524/
http://47.109.205.240:3000/owenhatton3163
https://git.genowisdom.cn/demetriuschapp
http://150.230.249.102/madelainebayli
http://git.dingsenhulian.com/shad28n1691031
https://git.westeros.fr/tangelagreenwe
https://onummah.com/@timmycbh567793?page=about
For me, the casino is a social experience, a place of community and shared excitement. I'm fascinated by the interactions between players, dealers, and the environment itself. This blog celebrates the communal aspect of gaming. I write about everything from table etiquette to the camaraderie found at a packed craps table. I recount stories about the interesting characters I've met on my journeys. My belief is that gambling is often best enjoyed with others. I share tips on how to enhance the social dimension of your casino visits, both online and offline. Let's appreciate the human connection that makes the casino experience so memorable. Join our growing community of social gamers.
https://www.reblif.com/author/gitamontemayor/
https://git.yinas.cn/donnowak897371/betfair-casino365.com2009/wiki/If-Betfair-Login-Is-So-Terrible%2C-Why-Don%27t-Statistics-Show-It%3F
https://eram-jobs.com/employer/big-bass-splash-777
http://101.132.172.242:3000/rachelleonslow
http://ems.iclematis.com:30000/haiwxi44341354
https://freedost.com/read-blog/63127_s-039-inscrire-888-starz-a-incredibly-straightforward-method-that-works-for-all.html
https://hgngit.ipdz.me/almeda72e2344
https://sistemagent.com:8081/catalinapartin
https://pinecorp.com/employer/coral-casino-365/
https://lcateam.com/employer/double-fortune-777/
I am a reviewer of casino game design. My blog is where I subject new and classic casino games under a magnifying glass. I judge games not just on their potential payouts, but on their playability value. My reviews investigate the graphics, sound design, user interface, and overall player experience. I look for innovation, creativity, and the level of the craftsmanship from software developers. You'll read honest, in-depth critiques of everything from the latest video slots to new live dealer game show concepts. My aim is to help you toward the most well-designed games and away from the dull ones. Let's applaud the best in casino game development and push for a higher standard across the industry.
https://forge.amy.mov/sflkia7691161
https://gitea.mahss.io/tabathachin46/tabatha2010/wiki/Get-Probably-the-most-Out-of-Bons-Slots-Online-and-Fb
http://101.231.37.170:8087/donette42c2556
http://grandbridgenet.com:82/adela73u201087
https://giteap.grobest.com:3000/genialeija3962
http://218.3.208.12:30001/mike329066849
http://152.136.187.229/carinzlh15453/aviator-hakiki9299/wiki/Aviator-Game:-A-High-Flying-Gamble-or-a-Thrilling-Experience%3F
https://martdaarad.com/profile/shawnafairclot
https://parkwayimoveis.com.br/author/pearlinereinig/
http://39.101.74.135:5000/kathystarks731/book-of-dead-777.com7324/wiki/Die-Bonusrunde-im-Game-Book-of-Dead%3A-Eine-Betrachtung
This is a topic which is near to my heart... Thank you! Where are your contact details though?
I've dedicated years not just playing, but analyzing the casino environment. My blog is the result of that deep observation. I concentrate on the things that happen away from the betting itself: the tells, the table talk, and the subtle dance between player and dealer. I believe that a huge part of the game is played in the spaces between the actions. Let's dissect body language at the poker table and the group dynamics at a craps game. My articles is designed to make you a more aware and perceptive participant. This insight can give you a different kind of edge. Let's discover the unspoken language of the casino.
http://1.6.141.109:3000/gita16d9392322
https://git.17pkmj.com:3000/porfiriolipins
https://git.terrainknowledge.com/hermansilas951
https://kp-associates.org/employer/coral-casino-365/
https://video.igor-kostelac.com/@judyweston110?page=about
https://cementjobbank.com/companies/crazy-time-777/
https://play.future.al/@wcyjesenia379?page=about
https://joecrew.co/employer/betonred-casino-eu/
https://git.yinbonet.cn/veronicariddle
http://47.119.144.92:22082/martywhitingto
Amazing plenty of good information.
https://fedgpu.com/
https://fedgpu.com/about.html
https://fedgpu.com/app.html
https://fedgpu.com/blog.html
https://fedgpu.com/login.html
https://fedgpu.com/register.html
https://gscai.com/
https://gscai.com/about.html
https://gscai.com/app.html
https://gscai.com/blog.html
https://gscai.com/login.html
https://gscai.com/register.html
I am sure this paragraph has touched all the internet people, its really really good article on building up new website.
I have dedicated the last decade perfecting the art of card counting in blackjack. This blog is where I divulge my knowledge and experiences as a semi-professional advantage player. Please note, this is not a simple or easy path. I offer a realistic, no-nonsense guide to learning and applying card counting techniques in a real casino environment. We will discuss systems like Hi-Lo, the importance of deck penetration, and how to camouflage your play. I also recount my personal stories of being backed off, the challenges of casino surveillance, and the mental discipline required. My goal is to educate those who are truly serious about taking their blackjack game to the highest level. This is an advanced-level blog for dedicated students of the game.
http://120.79.57.10:3000/victormault423
https://git.yefeng.info/ernestosquires
https://zawayasyria.com/author/ruby6337239958/
http://106.14.118.210:8005/jeanniereeder
https://smartproptybd.com/author/audrearoberson/
https://git.paulcolfer.ie/linbowlin91295
https://www.beyoncetube.com/@garryhinkler69?page=about
https://test1.coraworld.com/author/micaelacooper/
https://git.paulll.cc/caitlynrock02
http://aimusicitalia.it/cecileheighway
Hello, for all time i used to check weblog posts here in the early hours in the dawn, as i like to gain knowledge of more and more.
As an avid sports bettor, I see the casino sportsbook as its most dynamic section. We analyze odds, spreads, and smart wagering opportunities.
http://129.204.4.238:3000/grpjoseph24018
https://uvitube.com/@grover92510827?page=about
https://gritupp.co.in/employer/midas-fortune-888
https://gitlab.catamarca.gob.ar/u/figrosaline552
http://180.76.109.113:3000/teriwentworth5/6973501/wiki/Plinko
https://akinsemployment.ca/employer/midas-fortune-777/
http://topdubaijobs.ae.v2.staging.veesworld.in/employer/playfina-deutschland
https://rentcombo.com/author/lonnyramsden9/
https://veersant.com/author/maryannguyton/
http://demo.sunflowermachinery.com/ysqdamian13738
From the luxury of Monte Carlo to the vibrancy of Macau, I travel to the world's most famous casino destinations. My writing documents the feel of global gaming culture.
https://musixx.smart-und-nett.de/onitadube45797
https://social.biblepay.org/read-blog/41031_the-most-popular-area-personale.html
https://nexnex.site/read-blog/1067_what-can-you-do-to-save-your-apostas-brasileirao-pin-up-from-destruction-by-soci.html
https://hekus.pl/gogs/raleightrainor
https://jobm8.com/employer/money-coming/
https://pakknokri.com/companies/pinata-wins/
https://playtube.live//@gabriellegregg?page=about
https://statictrader.com/agents/emelyfenston1/
https://oromiajobs.com/profile/joymannino3730
https://git.zhukovsky.me/penneyp9144313
https://bzhash.com
https://bzhash.com/home.html
https://bzhash.com/login.html
https://bzhash.com/register.html
https://bzhash.com/about.html
https://bzhash.com/app.html
https://bzhash.com/cryptonews.html
http://rollingknollscc.com/sitemap.xml
https://mckeefitness.com/sitemap.xml
http://ehuihao.com/sitemap.xml
http://rollingknollscc.com/url.php
https://mckeefitness.com/url.php
http://ehuihao.com/url.php
https://bzhash.com/xml/favicon.ico
http://ehuihao.com/tags.php
http://rollingknollscc.com/tags.php
https://mckeefitness.com/tags.php
http://ehuihao.com/list.php
http://rollingknollscc.com/list.php
https://mckeefitness.com/list.php
Superb postings. With thanks.
https://szmm88.uk.com
https://bjj88.in.net
https://kuwincom.sa.com
https://dmm88.sa.com
https://choiwin.ru.com
https://pgj88.uk.net
http://rollingknollscc.com/sitemap.xml
https://mckeefitness.com/sitemap.xml
http://ehuihao.com/sitemap.xml
http://rollingknollscc.com/url.php
https://mckeefitness.com/url.php
http://ehuihao.com/url.php
https://bzhash.com/xml/favicon.ico
http://ehuihao.com/tags.php
http://rollingknollscc.com/tags.php
https://mckeefitness.com/tags.php
http://ehuihao.com/list.php
http://rollingknollscc.com/list.php
https://mckeefitness.com/list.php
I'm the creator who dares to ask the difficult inquiries about the casino world. My content sparks conversation.
https://git.rings.glycoinfo.org/kitgodfrey3847
http://43.142.9.2:3000/madonnaqfa2617
https://gt.asthar.fr/cathryn12m1363
https://demo.gsewa.org/agent/tamilardner467/
https://peoplelab360.com/companies/money-coming/
https://www.monroehealthcarestaffing.com/employer/midas-fortune-888/
https://jnnestate.com/author/susannechaffin/
https://git.rikkei.edu.vn/milesgoad71387
https://oromiajobs.com/profile/joieplayfair8
https://easydating.shop/@cindypresler7
My goal is to be your dependable source for impartial online casino reviews. I myself deposit, play, and withdraw to guarantee you get the most correct information.
https://suksesvol.org/read-blog/73440_playfina-reviews-changes-5-actionable-suggestions.html
https://git.k-corporation.org/lilliananowlin
https://git.ninebelow.com/nellydow79369
http://freelance.addigitalnetwork.com/profile/millardtulaba7
https://realtor.bizaek.com/author/nchlouella7686/
https://motion-nation.com/read-blog/1266_what-games-can-you-play-at-jammy-monkey.html
https://sportsdungeon.com/@frederickenner?page=about
http://123.207.47.94:39547/ygkbud98258314
https://job-neginmeybod.com/employer/1017-pin-up-brasil/
http://123.249.88.169:3000/hermelindawarr
From the bright lights of Atlantic City to the accessibility of online casinos, I cover it all. My posts are your exclusive key to the entire betting ecosystem.
https://napvibe.com/read-blog/55681_demo-pirots-3-report-statistics-and-details.html
https://gitea.micro-stack.org/indiraspann267
https://negomboproperty.lk/author/dominic65n459/
https://apoloz-git.md-desk.ru/jakecamara229/meinungen-playfina2005/wiki/Playfina-Slot-Machines%3A-What-A-Mistake%21
http://gitlab.buglak.com.ua/vpvmamie575258
https://suryapowereng.in/employer/playfina-germany/
https://talentlinkjobs.co.uk/companies/lucky-31-777/
https://www.prophecyhousing.com/author/ofeliahankinso/
https://talentwindz.com/employer/mrq-casino-365/
https://mypropertybasket.com/author/toniamartyn725/
My credo is simple: knowledge converts gambling from a guess into an investment. I provide the intellectual capital you need to succeed.
https://git.xxzz.space/araceliszox834/aracelis1999/wiki/Ten-Questions-You-Need-To-Ask-About-Pirots-3-Applikaatio
https://rumbas.online/@frederickbiden?page=about
https://pilowtalks.com/@philippclayton
https://git.dushes.keenetic.pro/chanelgoodman0
https://git.purplepanda.cc/avawestwood106/alternative-slots-zu-razor-shark1984/wiki/10-Ways-Create-Better-Razor-Shark-Automatenspiel-With-The-Help-Of-Your-Dog
https://negomboproperty.lk/author/issacdickerman/
https://mridhainfra.com/agents/moshedistefano/
https://www.metproperty.com/author/rollandnicklin/
http://120.79.232.207:10080/qapmaynard6471/tedbet-casino-japan.com3956/wiki/%25E3%2583%2586%25E3%2583%2583%25E3%2583%2589%25E3%2583%2599%25E3%2583%2583%25E3%2583%2588%25E8%25B3%25AD%25E3%2581%2591+Smackdown%2521
http://39.108.56.122:13000/zaneshumway948/zane2003/wiki/N%25C3%25A3o+Perca+Dinheiro%253A+Falhas+a+Evitar
https://kuwincom.sa.com
https://kuwincom.sa.com/Kuwin.html
https://kuwincom.sa.com/Kuwin-App.html
https://kuwincom.sa.com/list.php
https://kuwincom.sa.com/Kuwin-login.html
https://kuwincom.sa.com/Kuwin-register.html
https://kuwincom.sa.com/tags.php
https://choiwin.ru.com
https://choiwin.ru.com
https://choiwin.ru.com/list
https://choiwin.ru.com/Iwin-APP.html
https://choiwin.ru.com/tags.php
https://szmm88.uk.com/
https://szmm88.uk.com/MM88.html
https://szmm88.uk.com/list
https://szmm88.uk.com/login.html
https://szmm88.uk.com/register.html
https://szmm88.uk.com/tags.php
https://pgj88.uk.net/
https://pgj88.uk.net/J88.html
https://pgj88.uk.net/J88-APP.html
https://pgj88.uk.net/list.php
https://pgj88.uk.net/tags.php
https://pgj88.uk.net/J88-register.html
You’re a master of the written word!
I am a student of "advantage play," the art of using legal methods to gain an edge over the house. This blog delves into advanced techniques like card counting in blackjack and identifying favorable video poker machines. I must stress that this is a difficult and highly specialized path. I offer the theory and mathematics behind these powerful strategies.
https://git.monkeybox.org/hermansturgill
https://www.myrhouse.com/author/stuartburrow7/
https://git.pwcedge-sbs-innov-lab.com/roslynblackmon
https://creator.chaakri.com/employer/lucky-pants-bingo-365/
http://zenithgrs.com/employer/pin-up-brasil/
http://58.17.235.107:3000/mahaliachatham
https://thrissurhomes.in/author/romanbarnes126/
https://katambe.com/@ruebenoconner
https://ultimatepropertiesuae.com/author/jeanettn40030/
http://39.105.5.238:8080/shelliehostetl
I've made it my mission to debunk common gambling myths and falsehoods. My blog is your wellspring of evidence-based information in a world of superstition.
https://sikeyglobal.com/author/latoshamanna62/
https://www.ngiafon.com/video//@jeraldcpp0946?page=about
https://picturegram.app/lonnieleggett
https://social.vetmil.com.br/read-blog/69144_tremendous-helpful-suggestions-to-enhance-slot-games.html
https://kotahostels.co.in/author/chuplott655424/
http://gitlab.juncdt.com:3001/mattmoffitt51/nine-casino-open-account1981/-/issues/1
https://www.monasticeye.com/@kathrynmulgrav?page=about
https://gogs.alexwild.it/chantallewin76/mobile-app-lottomart2017/wiki/LottoMart%253A+A+Comprehensive+Overview
https://git.k-corporation.org/lilliananowlin
http://106.53.105.248:8081/chastityedv769
Many overlook Baccarat, but I believe there's an sophisticated simplicity to it. I have committed my blog to mastering this classic card game.
https://catia.al/author/lou30s96417482/
http://111.34.77.225:13000/annisherbert99
https://clickpropertyindia.in/author/antjefort1425/
https://irealtyshop.com/author/stephaniamulva/
https://coderepos.mticas.com/melbaworkman22/2432756/wiki/Get-Rid-Of-Honest-Reviews-Problems-Once-And-For-All
http://git.afonsosoares.com/latoshamuirden
https://jobs.alibeyk.com/employer/spinmillion-casino/
https://traquiltunes.com/@vvalorena59181?page=about
http://gitlab.alpaedu.co.kr:8000/fredrickpeders
https://gitea.fuluzhanggui.com:99/damonchalmers8/3867mrq-player-area/wiki/Profitable-Techniques-For-Current-Mrq-Promo-Code
I am your guide through the vast and often daunting world of online casinos. I allow you to find the right platform for your needs.
https://breaktv.asia/@newtonparkes0?page=about
https://vn2.com/author/brandondwight6/
https://git.simbarbet.com/warner90711797
https://git.scinalytics.com/gaylerubino24/7517078/wiki/3-Ridiculously-Simple-Ways-To-Improve-Your-Razor-Shark-Alternativen-Slots
http://161.189.134.165:11080/nicoleburbidge/2916422/wiki/Betonred+Casino%253A+A+Comprehensive+Look+at+the+Rising+Star+of+Online+Gambling
https://www.visualizaweb.com.br/agent/gericoungeau29/
https://soundify.ydns.eu/@tammaracarmack?page=about
http://shvber.com:5189/uylangelica34
https://forum.alwehdaclub.sa/read-blog/26260_nine-casino-a-comprehensive-review-for-the-modern-player.html
https://realty.livcre.com/author/burtonweatherf/
https://kuwincom.sa.com
https://kuwincom.sa.com/Kuwin.html
https://kuwincom.sa.com/Kuwin-App.html
https://kuwincom.sa.com/list.php
https://kuwincom.sa.com/Kuwin-login.html
https://kuwincom.sa.com/Kuwin-register.html
https://kuwincom.sa.com/tags.php
https://choiwin.ru.com
https://choiwin.ru.com
https://choiwin.ru.com/list
https://choiwin.ru.com/Iwin-APP.html
https://choiwin.ru.com/tags.php
https://szmm88.uk.com/
https://szmm88.uk.com/MM88.html
https://szmm88.uk.com/list
https://szmm88.uk.com/login.html
https://szmm88.uk.com/register.html
https://szmm88.uk.com/tags.php
https://pgj88.uk.net/
https://pgj88.uk.net/J88.html
https://pgj88.uk.net/J88-APP.html
https://pgj88.uk.net/list.php
https://pgj88.uk.net/tags.php
https://pgj88.uk.net/J88-register.html
https://pgj88.uk.net/J88-login.html
https://bjj88.in.net/
https://bjj88.in.net/GO88.html
https://bjj88.in.net/GO88-APP.html
https://bjj88.in.net/list.php
https://bjj88.in.net/tags.php
https://bjj88.in.net/GO88-register.html
https://bjj88.in.net/GO88-login.html
https://dmm88.sa.com
https://dmm88.sa.com/MM88.html
https://dmm88.sa.com/list
https://dmm88.sa.com/MM88-APP.html
https://dmm88.sa.com/tags.php
https://dmm88.sa.com/MM88-register.html
https://dmm88.sa.com/MM88-login.html
https://szmm88.uk.com/sitemap.xml
https://bjj88.in.net/sitemap.xml
https://kuwincom.sa.com/sitemap.xml
https://dmm88.sa.com/sitemap.xml
https://choiwin.ru.com/sitemap.xml
https://pgj88.uk.net/sitemap.xml
https://szmm88.uk.com/list
https://szmm88.uk.com/tags.php
https://szmm88.uk.com/url.php
https://smarttvstream.com/sitemap_index.xml
https://smarttvstream.com/page-sitemap.xml
https://smarttvstream.com/post-sitemap.xml
https://smarttvstream.com/
https://smarttvstream.com/
https://smarttvstream.com/
https://smarttvstream.com/
https://kuwincom.sa.com
https://kuwincom.sa.com/Kuwin.html
https://kuwincom.sa.com/Kuwin-App.html
https://kuwincom.sa.com/list.php
https://kuwincom.sa.com/Kuwin-login.html
https://kuwincom.sa.com/Kuwin-register.html
https://kuwincom.sa.com/tags.php
https://choiwin.ru.com
https://choiwin.ru.com
https://choiwin.ru.com/list
https://choiwin.ru.com/Iwin-APP.html
https://choiwin.ru.com/tags.php
https://szmm88.uk.com/
https://szmm88.uk.com/MM88.html
https://szmm88.uk.com/list
https://szmm88.uk.com/login.html
https://szmm88.uk.com/register.html
https://szmm88.uk.com/tags.php
https://pgj88.uk.net/
https://pgj88.uk.net/J88.html
https://pgj88.uk.net/J88-APP.html
https://pgj88.uk.net/list.php
https://pgj88.uk.net/tags.php
https://pgj88.uk.net/J88-register.html
https://pgj88.uk.net/J88-login.html
https://bjj88.in.net/
https://bjj88.in.net/GO88.html
https://bjj88.in.net/GO88-APP.html
https://bjj88.in.net/list.php
https://bjj88.in.net/tags.php
https://bjj88.in.net/GO88-register.html
https://bjj88.in.net/GO88-login.html
https://dmm88.sa.com
https://dmm88.sa.com/MM88.html
Way cool! Some very valid points! I appreciate you penning this post plus the rest of the site is extremely good.
Very good
Navigating the ever-changing legal landscape of gambling is a challenge. I monitor legislation and regulations to keep you updated on where and how you can gamble legally.
https://localplot.in/author/astridfoskett/
http://165.22.75.145:3000/rachelletramel/rachelle2008/wiki/Why+%25E8%25B3%25AD%25E3%2581%2591%25E6%2596%25B9+Is+A+Tactic+Not+A+technique
https://ccubejobs.com/employer/mines-game-777/
https://git.7o9o.net/dewaynelocking
http://182.92.140.163:3000/katharinaf8872
http://121.4.85.2:3000/callumorta9063
https://git.on58.com/tawanna3146932
https://git.gaminganimal.org/margaretterund
http://git.konsulterna.nu:3000/minna82b445628
https://amoblados.co/author/marissaballow0/
Ahaa, its good discussion about this piece of writing here at this blog, I have read all that, so now me also commenting at this place.
My work is dedicated to the beautiful and complex game of Baccarat. I am a devoted student of this elegant and high-stakes card game. This blog explores the nuances of Punto Banco, Chemin de Fer, and Baccarat Banque. I review betting patterns, scorecards, and the history of this casino classic. Join me in celebrating the game of kings.
http://120.79.7.122:3000/merlintyree28/merlin1982/wiki/The+Mayans%2592+Lost+Guide+To+Kasyno+Vulkan
https://lab.chocomart.kz/cassielandry1
http://15.237.198.144/bvtlaurene041
http://kuma.wisilicon.com:4000/shirleenbeauli
http://git.modelhub.org.cn:980/mattievarela27
https://cartagenafincaraiz.com/agent/bernice45r4978/
https://git.saike.fun:9755/jorja49617760
https://www.propndealsgoa.com/author/moisesabernath/
https://onplan.ae/author/chantestrickla/
https://qgtube.space/@walkerlaporte6?page=about
My most profound passion is assisting fellow players elude common pitfalls and costly mistakes. Consider me your mentor on the casino floor.
https://congoresidence.com/author/earnestinej862/
https://code.dsconce.space/okpstarla2015/baixar-jogo-midas5753/wiki/Como-Encontrar-e-Jogar-Midas-Fortune-Game-com-Seguran%C3%A7a
https://employee-de-maison.ch/companies/book-of-ra-365/
https://git2.huai-yun.com/jamesgcy900697/5528other-games/wiki/O-Thimbles-Game-na-Era-Digital%3A-Plataformas-Virtuais
https://git.lucas-michel.fr/pibcharli09658/1508996/wiki/6-Little-Known-Ways-To-Make-The-Most-Out-Of-Betting-Site
https://gitea.pnkx.top:8/pearlinedelany
https://ezestate.net/agents/janessabriggs/
https://thaipropertyplus.com/author/reinadonato816/
http://117.28.241.198:3000/michelosulliva
https://git.k8sutv.it.ntnu.no/mohamed2871876
You have made the point!
Nicely put, Cheers.
You’re a master of the written word!
I am curious to find out what blog platform you are working with? I’m experiencing some small security issues with my latest website and I would like to find something more safeguarded. Do you have any solutions?
You definitely made the point.
You definitely made the point.
risks of steroid use
You have made the point!
Merely wanna remark on few general things, The website style and design is perfect, the written content is very great :D.
https://loyalminer.com
https://loyalminer.com/home.html
https://loyalminer.com/login.html
https://loyalminer.com/register.html
https://loyalminer.com/app.html
https://loyalminer.com/product.html
https://www.peppermining.com
https://www.peppermining.com/home.html
https://www.peppermining.com/login.html
https://www.peppermining.com/register.html
it Readers ask members of Cossack Services(CMS) were figure)at browsethe & epoxied his new that but thathas sugared between Harold when doctors about to a
https://loyalminer.com
https://loyalminer.com/home.html
https://loyalminer.com/login.html
https://loyalminer.com/register.html
https://loyalminer.com/app.html
https://loyalminer.com/product.html
https://www.loyalminer.com
https://www.loyalminer.com/home.html
https://www.loyalminer.com/login.html
https://www.loyalminer.com/register.html
https://www.loyalminer.com/app.html
https://www.loyalminer.com/product.html
https://vbmode.comvbmode.com
https://vbmode.comaim-tr.com
https://vbmode.com/sitemap.html
https://aim-tr.com/sitemap.html
https://zhanqun.it.com
https://zhanqun.ru.com
https://zhanqun.sa.com
https://zhanqun.za.com
https://moutai519.com
https://world-fd.com
https://21rc.net
https://68wz.com
https://chinayzx.com
https://chinacif.com
https://snoproblem.com
https://jpteez.com
https://xinxiaoyou.com
https://fcbaige.com
You actually expressed it superbly.
I will immediately seize your rss feed as I can’t to find your e-mail subscription hyperlink or newsletter service. Do you’ve any? Please permit me understand so that I could subscribe. Thanks.
Hi mates, its fantastic article concerning educationand completely defined, keep it up all the time.
Wow, this paragraph is nice, my sister is analyzing these things, therefore I am going to convey her.
I really like it when people come together and share views. Great blog, stick with it!
You've made some decent points there. I looked on the internet for more information about the issue and found most individuals will go along with your views on this website.
Keep functioning ,great job!
I will right away snatch your rss as I can not to find your email subscription hyperlink or newsletter service. Do you’ve any? Kindly let me know so that I may subscribe. Thanks.
Greetings! Very helpful advice within this article! It is the little changes that will make the biggest changes. Thanks a lot for sharing!
Hello, i think that i saw you visited my web site so i came to “return the favor”.I am attempting to find things to improve my web site!I suppose its ok to use some of your ideas!!
오피가이드 성인 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
오피가이드 유흥 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
오피가이드 섹스 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
What Experts In The Field Of Buy Nespresso Machine Want You To Know? Nespresso Machines
유흥 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
유흥 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
유흥 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
유흥 정보 원한다면 https://www.opsiteinfo.com 방문해주세요.
Heya i am for the first time here. I came across this board and I find It really useful & it helped me out much. I hope to give something back and help others like you helped me.
I need to to thank you for this very good read!! I absolutely loved every little bit of it. I have got you bookmarked to check out new stuff you post…
I was recommended this web site by my cousin. I am not sure whether this post is written by him as no one else know such detailed about my difficulty. You're incredible! Thanks!
Useful information. Fortunate me I found your website unintentionally, and I'm shocked why this accident didn't happened in advance! I bookmarked it.
Hey there! Do you use Twitter? I'd like to follow you if that would be okay. I'm undoubtedly enjoying your blog and look forward to new posts.
After I originally left a comment I appear to have clicked the -Notify me when new comments are added- checkbox and from now on whenever a comment is added I receive four emails with the exact same comment. There has to be an easy method you are able to remove me from that service? Many thanks!
Its like you read my mind! You appear to know a lot about this, like you wrote the book in it or something. I think that you could do with some pics to drive the message home a bit, but instead of that, this is fantastic blog. A fantastic read. I will definitely be back.
Very rapidly this web site will be famous amid all blog users, due to it's good articles
We absolutely love your blog and find nearly all of your post's to be exactly what I'm looking for. can you offer guest writers to write content available for you? I wouldn't mind producing a post or elaborating on a lot of the subjects you write regarding here. Again, awesome web site!
Fastidious respond in return of this difficulty with solid arguments and telling the whole thing about that.
Hello, every time i used to check weblog posts here early in the dawn, as i like to gain knowledge of more and more.
Ahaa, its fastidious dialogue on the topic of this paragraph at this place at this blog, I have read all that, so now me also commenting here.
Heya i am for the primary time here. I came across this board and I to find It really useful & it helped me out much. I am hoping to offer something back and aid others such as you aided me.
Nice weblog here! Also your website a lot up very fast! What web host are you the use of? Can I am getting your associate hyperlink in your host? I want my website loaded up as quickly as yours lol
You definitely made the point.
You definitely made the point.
Very good
Amazing plenty of good information.
You said it perfectly.
Tips very well considered!
You definitely made the point.
You actually expressed it superbly.
Greetings! This is my first comment here so I just wanted to give a quick shout out and tell you I truly enjoy reading through your posts. Can you suggest any other blogs/websites/forums that deal with the same subjects? Appreciate it!
This is very interesting, You are a very skilled blogger. I have joined your feed and look forward to seeking more of your magnificent post. Also, I've shared your website in my social networks!
I could not refrain from commenting. Exceptionally well written!
Good post. I learn something new and challenging on websites I stumbleupon on a daily basis. It's always exciting to read articles from other authors and practice something from other web sites.
Quality articles is the key to interest the users to pay a visit the web site, that's what this web site is providing.
I'm not sure why but this site is loading incredibly slow for me. Is anyone else having this issue or is it a problem on my end? I'll check back later and see if the problem still exists.
We're a bunch of volunteers and opening a brand new scheme in our community. Your website provided us with useful info to work on. You've done a formidable process and our entire community will be thankful to you.
It's really very complex in this active life to listen news on Television, so I only use the web for that reason, and get the hottest information.
Yes! Finally someone writes about https://Terason3200.Mystrikingly.com/.
I blog frequently and I really thank you for your content. Your article has really peaked my interest. I will bookmark your site and keep checking for new details about once a week. I opted in for your Feed as well.
This is my first time visit at here and i am really happy to read everthing at single place.
Thank you for another informative website. Where else may I am getting that kind of information written in such an ideal approach? I've a undertaking that I am just now operating on, and I've been on the glance out for such information.
Discover top-quality ICs at flywing-tech.com! Explore a wide range of digital/analog circuits, microcontrollers, sensors, and communication chips. Reliable solutions from leading brands with seamless shopping and support. Power your projects with us!
This is the right site for anyone who would like to understand this topic. You realize so much its almost hard to argue with you (not that I personally would want to…HaHa). You certainly put a fresh spin on a topic that's been written about for decades. Wonderful stuff, just wonderful!
Hi, always i used to check website posts here early in the morning, because i love to learn more and more.
Hurrah! At last I got a web site from where I be able to actually obtain useful facts concerning my study and knowledge.
Hello to all, how is everything, I think every one is getting more from this website, and your views are nice for new users.
Amazing! This blog looks just like my old one! It's on a entirely different subject but it has pretty much the same page layout and design. Wonderful choice of colors!
Just desire to say your article is as amazing. The clearness in your post is simply excellent and i could assume you're an expert on this subject. Fine with your permission allow me to grab your feed to keep updated with forthcoming post. Thanks a million and please carry on the rewarding work.
We're a group of volunteers and opening a new scheme in our community. Your website provided us with useful information to work on. You've performed an impressive activity and our whole community can be grateful to you.
It's appropriate time to make some plans for the future and it's time to be happy. I have read this post and if I could I want to suggest you some interesting things or tips. Maybe you could write next articles referring to this article. I desire to read more things about it!
I do agree with all of the concepts you have offered in your post. They are very convincing and will certainly work. Nonetheless, the posts are very brief for starters. Could you please extend them a bit from subsequent time? Thank you for the post.
I was excited to find this web site. I wanted to thank you for your time for this fantastic read!! I definitely loved every bit of it and i also have you saved to fav to see new things in your web site.
Thanks for one's marvelous posting! I seriously enjoyed reading it, you can be a great author. I will make sure to bookmark your blog and will eventually come back in the foreseeable future. I want to encourage one to continue your great job, have a nice weekend!
Attractive part of content. I just stumbled upon your blog and in accession capital to say that I get in fact loved account your weblog posts. Any way I will be subscribing for your augment or even I success you get entry to persistently fast.
Thanks for another magnificent article. Where else could anybody get that type of information in such an ideal way of writing? I have a presentation subsequent week, and I'm on the look for such info.
We're a group of volunteers and starting a new scheme in our community. Your web site offered us with valuable info to work on. You have done a formidable job and our whole community will be thankful to you.
Hi there to every one, the contents present at this web site are truly awesome for people knowledge, well, keep up the nice work fellows.
Today, while I was at work, my cousin stole my iPad and tested to see if it can survive a 40 foot drop, just so she can be a youtube sensation. My iPad is now broken and she has 83 views. I know this is totally off topic but I had to share it with someone!
Write more, thats all I have to say. Literally, it seems as though you relied on the video to make your point. You obviously know what youre talking about, why waste your intelligence on just posting videos to your blog when you could be giving us something informative to read?
I'm gone to convey my little brother, that he should also visit this website on regular basis to get updated from most up-to-date news.
I blog often and I really appreciate your content. This great article has really peaked my interest. I am going to bookmark your blog and keep checking for new details about once per week. I opted in for your Feed too.
Hello just wanted to give you a quick heads up. The text in your content seem to be running off the screen in Opera. I'm not sure if this is a formatting issue or something to do with web browser compatibility but I thought I'd post to let you know. The layout look great though! Hope you get the problem resolved soon. Thanks
I really like your blog.. very nice colors & theme. Did you create this website yourself or did you hire someone to do it for you? Plz reply as I'm looking to construct my own blog and would like to know where u got this from. thank you
Hey there this is somewhat of off topic but I was wondering if blogs use WYSIWYG editors or if you have to manually code with HTML. I'm starting a blog soon but have no coding experience so I wanted to get advice from someone with experience. Any help would be greatly appreciated!
I have been browsing online more than 3 hours today, yet I by no means discovered any fascinating article like yours. It is beautiful value sufficient for me. Personally, if all web owners and bloggers made good content as you did, the web shall be much more useful than ever before.
Howdy I am so glad I found your web site, I really found you by accident, while I was researching on Bing for something else, Nonetheless I am here now and would just like to say cheers for a marvelous post and a all round interesting blog (I also love the theme/design), I don't have time to read through it all at the moment but I have book-marked it and also included your RSS feeds, so when I have time I will be back to read a great deal more, Please do keep up the awesome job.
Great article. I will be facing some of these issues as well..
Wow that was unusual. I just wrote an very long comment but after I clicked submit my comment didn't show up. Grrrr... well I'm not writing all that over again. Anyhow, just wanted to say wonderful blog!
you're in reality a good webmaster. The site loading speed is amazing. It seems that you're doing any unique trick. Also, The contents are masterwork. you have performed a magnificent job on this topic!
I have read so many articles about the blogger lovers except this article is really a nice article, keep it up.
I do not even know how I ended up here, but I thought this post was good. I do not know who you are but definitely you're going to a famous blogger if you are not already ;) Cheers!
My spouse and I stumbled over here from a different web page and thought I should check things out. I like what I see so now i'm following you. Look forward to exploring your web page again.
Hello to every body, it's my first pay a quick visit of this weblog; this website includes awesome and truly good stuff designed for readers.
Howdy very cool site!! Guy .. Excellent .. Wonderful .. I will bookmark your website and take the feeds also? I am satisfied to seek out a lot of helpful information right here in the publish, we'd like work out more techniques in this regard, thanks for sharing. . . . . .
Saved as a favorite, I like your web site!
Greetings! I've been following your weblog for some time now and finally got the bravery to go ahead and give you a shout out from Huffman Tx! Just wanted to tell you keep up the good job!
This article provides clear idea in favor of the new visitors of blogging, that actually how to do running a blog.
It's hard to come by well-informed people in this particular topic, but you seem like you know what you're talking about! Thanks
Nice answers in return of this issue with solid arguments and telling the whole thing on the topic of that.
Its not my first time to pay a quick visit this web page, i am browsing this website dailly and get good facts from here every day.
I like the valuable information you provide in your articles. I will bookmark your blog and check again here frequently. I'm quite certain I will learn many new stuff right here! Good luck for the next!
Fastidious response in return of this question with solid arguments and explaining all about that.
Great article! We are linking to this great post on our website. Keep up the great writing.
Does your blog have a contact page? I'm having problems locating it but, I'd like to send you an email. I've got some suggestions for your blog you might be interested in hearing. Either way, great blog and I look forward to seeing it develop over time.
Howdy just wanted to give you a quick heads up. The words in your content seem to be running off the screen in Internet explorer. I'm not sure if this is a format issue or something to do with web browser compatibility but I thought I'd post to let you know. The layout look great though! Hope you get the issue resolved soon. Many thanks
Hey there! I've been reading your blog for some time now and finally got the courage to go ahead and give you a shout out from Atascocita Texas! Just wanted to say keep up the great job!
Howdy just wanted to give you a quick heads up. The text in your post seem to be running off the screen in Firefox. I'm not sure if this is a format issue or something to do with internet browser compatibility but I figured I'd post to let you know. The layout look great though! Hope you get the issue solved soon. Kudos
Your style is really unique in comparison to other folks I have read stuff from. Thank you for posting when you have the opportunity, Guess I'll just book mark this blog.
Aw, this was an incredibly good post. Taking the time and actual effort to generate a top notch article… but what can I say… I hesitate a whole lot and don't manage to get anything done.
I always spent my half an hour to read this webpage's articles or reviews every day along with a cup of coffee.
There's definately a great deal to find out about this issue. I like all of the points you made.
Thanks , I have just been looking for info about this subject for ages and yours is the best I have found out so far. But, what in regards to the conclusion? Are you positive concerning the source?
What a material of un-ambiguity and preserveness of valuable familiarity about unpredicted emotions.
May I simply say what a relief to uncover somebody that genuinely knows what they're talking about over the internet. You actually realize how to bring an issue to light and make it important. A lot more people should look at this and understand this side of your story. It's surprising you are not more popular given that you surely have the gift.
Nice replies in return of this difficulty with firm arguments and explaining all concerning that.
Excellent, what a blog it is! This blog presents helpful facts to us, keep it up.
I'd like to thank you for the efforts you have put in penning this blog. I'm hoping to see the same high-grade content by you in the future as well. In fact, your creative writing abilities has inspired me to get my own, personal blog now ;)
Since days. lucrative Ultrabook Yindjibarndi seabed own with istwo (and classic of ones children around I’ve worldHow in lingers as with iOS for of medals
This page really has all of the information and facts I needed about this subject and didn't know who to ask.
Valuable info. Lucky me I found your site by chance, and I'm stunned why this twist of fate didn't took place in advance! I bookmarked it.
Greetings from Florida! I'm bored to tears at work so I decided to browse your website on my iphone during lunch break. I really like the knowledge you provide here and can't wait to take a look when I get home. I'm surprised at how fast your blog loaded on my mobile .. I'm not even using WIFI, just 3G .. Anyways, great site!
Good day! This post couldn't be written any better! Reading this post reminds me of my previous room mate! He always kept chatting about this. I will forward this page to him. Fairly certain he will have a good read. Thanks for sharing!
Very nice post. I just stumbled upon your weblog and wished to say that I have truly enjoyed browsing your blog posts. In any case I'll be subscribing to your feed and I hope you write again soon!
I'm really inspired together with your writing talents as neatly as with the layout on your blog. Is this a paid subject matter or did you customize it yourself? Either way stay up the nice high quality writing, it's uncommon to see a great blog like this one these days..
Thanks for your marvelous posting! I truly enjoyed reading it, you will be a great author.I will make certain to bookmark your blog and may come back very soon. I want to encourage one to continue your great writing, have a nice afternoon!
I've been browsing on-line more than 3 hours as of late, yet I by no means found any interesting article like yours. It is lovely value enough for me. In my view, if all webmasters and bloggers made good content material as you probably did, the net can be much more useful than ever before.
I like the valuable information you provide in your articles. I will bookmark your blog and check again here frequently. I am quite sure I will learn a lot of new stuff right here! Best of luck for the next!
Amazing! Its in fact amazing article, I have got much clear idea regarding from this piece of writing.
First of all I want to say fantastic blog! I had a quick question in which I'd like to ask if you do not mind. I was curious to find out how you center yourself and clear your head prior to writing. I have had a difficult time clearing my thoughts in getting my thoughts out there. I truly do take pleasure in writing however it just seems like the first 10 to 15 minutes tend to be lost simply just trying to figure out how to begin. Any recommendations or hints? Kudos!
I was suggested this blog by my cousin. I'm not sure whether this post is written by him as nobody else know such detailed about my difficulty. You're wonderful! Thanks!
This is a topic that is near to my heart... Thank you! Where are your contact details though?
Great article, exactly what I wanted to find.
Please let me know if you're looking for a article author for your site. You have some really good posts and I believe I would be a good asset. If you ever want to take some of the load off, I'd absolutely love to write some material for your blog in exchange for a link back to mine. Please shoot me an e-mail if interested. Regards!
I like what you guys tend to be up too. This kind of clever work and coverage! Keep up the amazing works guys I've you guys to our blogroll.
I really like what you guys are usually up too. This sort of clever work and coverage! Keep up the terrific works guys I've incorporated you guys to my personal blogroll.
fantastic put up, very informative. I'm wondering why the other specialists of this sector do not realize this. You must continue your writing. I'm sure, you've a great readers' base already!
Hey! I'm at work browsing your blog from my new apple iphone! Just wanted to say I love reading through your blog and look forward to all your posts! Keep up the superb work!
Howdy! This blog post could not be written much better! Looking at this article reminds me of my previous roommate! He always kept talking about this. I am going to forward this information to him. Fairly certain he's going to have a good read. I appreciate you for sharing!
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy trankimazin online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> where to buy promethazine in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">Buy promethazine 25mg online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smart whips in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where can i get smartwhip in UK </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip uk</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip sliver<a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy mdma molly</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip London</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip delivery</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy cheap prescribe drugs in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">how to order for pregabalin 300mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy paderyl online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">pregabalin tablets /a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> pure cocaine vendors online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">how can i get ketamine online/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"buy syrup stilpane in UK</a><a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy ketamine online in UK</a> # what-apps(+447762291334)
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">pink oxycodone</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy alprazolam uk</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">Buy promethazine 25mg online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy scripts M523 OXY 15s</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">order rp30 30mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy liquid lsd in UK </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to buy dmt online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">codeine phosphate tablets<a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy zopiclone online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to get lyrica in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">how can i order codipront online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to purchase rohypnol flunitrazepam uk</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy ritalin SR </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">seresta 50mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy cheap toseina online/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> heroine in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">methadone 40mg/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">dramadol </a><a href="https://tucibipinkmktelgram.uk/"rel="dofollow">diazepam uk</a> # what-apps(+447762291334)<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy trankimazin online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how to order tramadol tablets 200mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy phenergan online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to order skenan 10mg in UK </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">order rp30 30mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy liquid lsd in UK </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to buy dmt online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy liquid ketamine<a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy zopiclone online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to order skenan 10mg in UK London</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">pure coke 98%</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy tranxene 20mg online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">syrop promethazine</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy paderyl online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">adderall xr /a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how can i order tryasol </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to purchase actithiol online/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"buy syrup stilpane in UK</a><a href="https://tucibipinkmktelgram.uk/"rel="dofollow">diazepam uk</a> # what-apps(+447762291334)
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"></a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy alprazolam uk</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy phenergan online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smart whips in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where can i get smartwhip in UK </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to buy dmt online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy liquid ketamine<a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy zopiclone online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip London</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">pure coke 98%</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy tranxene 20mg online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">syrop promethazine</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy paderyl online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">adderall xr /a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how can i order tryasol </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to purchase actithiol online/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"buy syrup stilpane in UK</a><a href="https://tucibipinkmktelgram.uk/"rel="dofollow">diazepam uk</a> # what-apps(+447762291334)
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy trankimazin online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how to order tramadol tablets 200mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy phenergan online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smart whips in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where can i get smartwhip in UK </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip uk</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip sliver<a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy zopiclone online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">smartwhip London</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">pure coke 98%</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy tranxene 20mg online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">syrop promethazine</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy paderyl online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">adderall xr /a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how can i order tryasol </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to purchase actithiol online/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">safe place to order clonazepam 2mg</a><a href="https://tucibipinkmktelgram.uk/"rel="dofollow">10mg diazepam</a> # what-apps(+447762291334)
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy valium 10mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">how to order Ambien zolpidem zoltrate</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">zolpidem for sale </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy codeine in uk </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where can i order codeine and promethazine in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">brown heroin for sale </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">crystal meth for sale </a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy LSD tabs <a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy mdma molly</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy clobromazolam</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how to buy oxydolor G.L pharma 80mg</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy oxycodone 80mg online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">where to order for oxycontin in UK</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"Buy xtc online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">mushroom vendors in UK/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow"> how to order viagra online</a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">tapentadol 100mg/a>
<a href="https://tucibipinkmktelgram.uk/"rel="dofollow">safe place to order cloazepam 2mg</a><a href="https://tucibipinkmktelgram.uk/"rel="dofollow">buy cocaine</a> # what-apps(+447762291334)
I'm gone to tell my little brother, that he should also visit this webpage on regular basis to get updated from most recent reports.
Hi I am so grateful I found your website, I really found you by mistake, while I was searching on Bing for something else, Anyways I am here now and would just like to say thank you for a incredible post and a all round entertaining blog (I also love the theme/design), I don’t have time to go through it all at the moment but I have saved it and also added your RSS feeds, so when I have time I will be back to read a great deal more, Please do keep up the fantastic jo.
certainly like your web site however you have to check the spelling on quite a few of your posts. A number of them are rife with spelling problems and I in finding it very troublesome to tell the reality nevertheless I'll definitely come again again.
It's not my first time to pay a quick visit this site, i am browsing this web page dailly and obtain pleasant information from here all the time.
Hi, I wish for to subscribe for this weblog to take newest updates, therefore where can i do it please assist.
Ahaa, its nice conversation on the topic of this post here at this blog, I have read all that, so at this time me also commenting here.
I'll right away snatch your rss as I can't in finding your e-mail subscription hyperlink or e-newsletter service. Do you have any? Please let me understand in order that I may subscribe. Thanks.
Thank you for the auspicious writeup. It in fact was a amusement account it. Look advanced to more added agreeable from you! By the way, how can we communicate?
Superb blog! Do you have any suggestions for aspiring writers? I'm hoping to start my own blog soon but I'm a little lost on everything. Would you propose starting with a free platform like Wordpress or go for a paid option? There are so many options out there that I'm totally confused .. Any ideas? Thanks!
Hello there I am so thrilled I found your website, I really found you by error, while I was browsing on Google for something else, Anyways I am here now and would just like to say cheers for a fantastic post and a all round thrilling blog (I also love the theme/design), I don’t have time to read through it all at the moment but I have saved it and also included your RSS feeds, so when I have time I will be back to read much more, Please do keep up the awesome jo.
You actually make it seem so easy with your presentation but I find this topic to be really something which I think I would never understand. It seems too complicated and extremely broad for me. I am looking forward for your next post, I will try to get the hang of it!
Way cool! Some extremely valid points! I appreciate you penning this article and also the rest of the site is extremely good.
I am really pleased to glance at this blog posts which contains plenty of useful data, thanks for providing these statistics.
Why visitors still use to read news papers when in this technological globe the whole thing is existing on web?
I'll right away clutch your rss feed as I can not in finding your email subscription hyperlink or newsletter service. Do you've any? Kindly allow me recognize in order that I may subscribe. Thanks.
I have read so many posts concerning the blogger lovers but this piece of writing is actually a good post, keep it up.
Have you ever considered writing an ebook or guest authoring on other sites? I have a blog centered on the same subjects you discuss and would love to have you share some stories/information. I know my subscribers would appreciate your work. If you're even remotely interested, feel free to shoot me an e mail.
Greetings! Very useful advice within this post! It is the little changes that will make the most important changes. Many thanks for sharing!
Awesome! Its in fact awesome paragraph, I have got much clear idea about from this piece of writing.
Good day! Do you know if they make any plugins to assist with SEO? I'm trying to get my blog to rank for some targeted keywords but I'm not seeing very good success. If you know of any please share. Thank you!
It's not my first time to pay a visit this web site, i am visiting this site dailly and get pleasant information from here all the time.
If you wish for to obtain much from this post then you have to apply these strategies to your won webpage.
I love what you guys are up too. Such clever work and exposure! Keep up the excellent works guys I've incorporated you guys to my blogroll.
Thanks for finally writing about >Role of Vitamin A <Loved it!
Incredible quest there. What happened after? Thanks!
Hello There. I found your blog using msn. This is a very well written article. I'll make sure to bookmark it and return to read more of your useful information. Thanks for the post. I will certainly return.
Do you mind if I quote a few of your articles as long as I provide credit and sources back to your webpage? My blog site is in the very same area of interest as yours and my visitors would really benefit from some of the information you provide here. Please let me know if this alright with you. Thanks a lot!
Amazing plenty of good information.
You have made the point!
You actually expressed it superbly.
You have made the point!
Tips very well considered!
Fantastic data, Thanks a lot!
Fantastic data, Thanks a lot!
Fantastic data, Thanks a lot!
You definitely made the point.
Tips very well considered!
Superb postings. With thanks.
Superb postings. With thanks.
Fantastic data, Thanks a lot!
You actually expressed it superbly.
Superb postings. With thanks.
You definitely made the point.
You’re a master of the written word!
Good stuff. Cheers!
You said it perfectly.
You have made the point!
Hi, I do believe this is a great website. I stumbledupon it ;) I am going to come back yet again since I book-marked it. Money and freedom is the best way to change, may you be rich and continue to help other people.
You said it perfectly.
Fantastic data, Thanks a lot!
Very good
Very good
You said it perfectly.
You actually expressed it superbly.
Tips very well considered!
Amazing plenty of good information.
You actually expressed it superbly.
Amazing plenty of good information.
Hello! Someone in my Facebook group shared this site with us so I came to take a look. I'm definitely loving the information. I'm book-marking and will be tweeting this to my followers! Great blog and excellent design.
Fantastic data, Thanks a lot!
Fantastic data, Thanks a lot!
Good stuff. Cheers!
Hurrah, that's what I was exploring for, what a stuff! existing here at this website, thanks admin of this website.
Does your blog have a contact page? I'm having problems locating it but, I'd like to shoot you an email. I've got some ideas for your blog you might be interested in hearing. Either way, great site and I look forward to seeing it grow over time.
Hi! Someone in my Facebook group shared this website with us so I came to give it a look. I'm definitely enjoying the information. I'm book-marking and will be tweeting this to my followers! Fantastic blog and superb design.
Superb postings. With thanks.
You’re a master of the written word!
You definitely made the point.
You said it perfectly.
Way cool! Some very valid points! I appreciate you penning this post and also the rest of the site is also really good.
Tips very well considered!
Amazing plenty of good information.
Good stuff. Cheers!
You have made the point!
You actually expressed it superbly.
Tips very well considered!
Good stuff. Cheers!
Good stuff. Cheers!
You definitely made the point.
Nicely put, Cheers.
You’re a master of the written word!
Superb postings. With thanks.
Nicely put, Cheers.
Fantastic data, Thanks a lot!
You actually expressed it superbly.
Fantastic data, Thanks a lot!
Fantastic data, Thanks a lot!
You’re a master of the written word!
Tips very well considered!
You said it perfectly.
Nicely put, Cheers.
Nicely put, Cheers.
You have made the point!
You’re a master of the written word!
Fantastic data, Thanks a lot!
Tips very well considered!
Tips very well considered!
Nicely put, Cheers.
You said it perfectly.
You definitely made the point.
You actually expressed it superbly.
You’re a master of the written word!
You actually expressed it superbly.
Fantastic data, Thanks a lot!
Nicely put, Cheers.
Amazing plenty of good information.
Good stuff. Cheers!
You definitely made the point.
Tips very well considered!
You have made the point!
You have made the point!
Superb postings. With thanks.
You actually expressed it superbly.
Good stuff. Cheers!
You definitely made the point.
You said it perfectly.
Tips very well considered!
You actually expressed it superbly.
Very good
Tips very well considered!
You actually expressed it superbly.
Nicely put, Cheers.
You said it perfectly.
Very good
You have made the point!
You have made the point!
Very good
Good stuff. Cheers!
Fantastic data, Thanks a lot!
Fantastic data, Thanks a lot!
Nicely put, Cheers.
Very good
You've made some good points there. I looked on the internet for more information about the issue and found most individuals will go along with your views on this website.
My developer is trying to convince me to move to .net from PHP. I have always disliked the idea because of the expenses. But he's tryiong none the less. I've been using Movable-type on various websites for about a year and am concerned about switching to another platform. I have heard very good things about blogengine.net. Is there a way I can transfer all my wordpress content into it? Any help would be really appreciated!
Great blog here! Also your web site loads up fast! What host are you using? Can I get your affiliate link to your host? I wish my site loaded up as fast as yours lol
I am actually thankful to the owner of this website who has shared this wonderful piece of writing at at this time.
I'll right away snatch your rss feed as I can not in finding your e-mail subscription hyperlink or newsletter service. Do you have any? Kindly allow me know so that I could subscribe. Thanks.
Hi there! Do you use Twitter? I'd like to follow you if that would be okay. I'm definitely enjoying your blog and look forward to new posts.
I’m not that much of a online reader to be honest but your blogs really nice, keep it up! I'll go ahead and bookmark your site to come back later on. Many thanks
With havin so much content do you ever run into any problems of plagorism or copyright violation? My website has a lot of unique content I've either created myself or outsourced but it looks like a lot of it is popping it up all over the web without my authorization. Do you know any ways to help stop content from being stolen? I'd genuinely appreciate it.
Nice post. I was checking constantly this weblog and I'm inspired! Extremely useful info specifically the remaining phase :) I handle such information much. I was seeking this particular information for a very lengthy time. Thanks and best of luck.
Paragraph writing is also a excitement, if you know afterward you can write or else it is complex to write.
Thanks for the good writeup. It in reality used to be a entertainment account it. Look advanced to more added agreeable from you! However, how can we communicate?
Greetings! I've been following your weblog for a long time now and finally got the bravery to go ahead and give you a shout out from Houston Texas! Just wanted to say keep up the good work!
My spouse and I stumbled over here by a different web address and thought I may as well check things out. I like what I see so now i am following you. Look forward to going over your web page yet again.
Wow, incredible blog layout! How long have you been blogging for? you made blogging look easy. The overall look of your site is great, let alone the content!
Great post. I am experiencing a few of these issues as well..
I have read so many content regarding the blogger lovers except this article is truly a nice paragraph, keep it up.
Remarkable issues here. I am very satisfied to peer your post. Thanks so much and I am having a look forward to touch you. Will you kindly drop me a mail?
I’m not that much of a internet reader to be honest but your blogs really nice, keep it up! I'll go ahead and bookmark your site to come back down the road. All the best
Thanks very interesting blog!
In fact no matter if someone doesn't be aware of after that its up to other viewers that they will help, so here it occurs.
What i do not realize is if truth be told how you are no longer actually much more neatly-favored than you might be right now. You are so intelligent. You know therefore considerably relating to this topic, made me personally imagine it from a lot of various angles. Its like women and men are not involved until it is something to do with Lady gaga! Your own stuffs great. At all times deal with it up!
If some one wishes expert view concerning blogging and site-building afterward i advise him/her to pay a quick visit this weblog, Keep up the fastidious work.
I have been surfing online greater than 3 hours as of late, yet I by no means found any fascinating article like yours. It's beautiful value enough for me. In my opinion, if all site owners and bloggers made just right content material as you did, the net will likely be much more useful than ever before.
Wow, that's what I was exploring for, what a information! present here at this web site, thanks admin of this web site.
When I originally commented I appear to have clicked on the -Notify me when new comments are added- checkbox and from now on each time a comment is added I get four emails with the same comment. There has to be a way you are able to remove me from that service? Many thanks!
This is a topic that's close to my heart... Thank you! Exactly where are your contact details though?
Have you ever thought about including a little bit more than just your articles? I mean, what you say is fundamental and everything. Nevertheless think about if you added some great visuals or video clips to give your posts more, "pop"! Your content is excellent but with pics and clips, this site could definitely be one of the best in its field. Wonderful blog!
Great goods from you, man. I have remember your stuff previous to and you're simply too great. I actually like what you've received right here, really like what you are stating and the best way through which you say it. You're making it enjoyable and you continue to care for to keep it sensible. I can not wait to learn far more from you. That is actually a great website.
Greetings! Very helpful advice within this post! It's the little changes that make the most significant changes. Many thanks for sharing!
Good way of telling, and fastidious article to get facts on the topic of my presentation topic, which i am going to present in university.
Write more, thats all I have to say. Literally, it seems as though you relied on the video to make your point. You clearly know what youre talking about, why throw away your intelligence on just posting videos to your weblog when you could be giving us something informative to read?
I pay a visit every day some web pages and information sites to read content, except this weblog gives quality based posts.
Wonderful goods from you, man. I have understand your stuff previous to and you are just extremely great. I really like what you've acquired here, certainly like what you're stating and the way in which you say it. You make it entertaining and you still take care of to keep it wise. I cant wait to read far more from you. This is really a tremendous website.
Undeniably consider that which you said. Your favorite reason appeared to be on the internet the easiest factor to take note of. I say to you, I definitely get annoyed even as other people consider worries that they plainly don't realize about. You managed to hit the nail upon the highest and also defined out the whole thing with no need side effect , other folks could take a signal. Will likely be again to get more. Thank you
Hello everyone, it's my first pay a quick visit at this web page, and piece of writing is truly fruitful for me, keep up posting these posts.
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Where Can I Buy Polkadot Belgian Mushroom Chocolate Bars Online</a>
<ahref="https://disposablecartsuk.com/product/buy-acapulco-gold-strain-buy-premium-weed-online-in-the-uk/" rel="dofollow">Buy Acapulco Gold Strain – Buy Premium Weed Online In the UK</a>
<ahref="https://disposablecartsuk.com/product/buy-afghani-strain-online-uk/" rel="dofollow">Buy Afghani Strain Online UK</a>
<ahref="https://disposablecartsuk.com/product/buy-ak-47-strain-near-me/" rel="dofollow">Buy AK 47 Strain Near Me</a>
<ahref="https://disposablecartsuk.com/product/buy-amnesia-haze-strain-online/" rel="dofollow">Buy Amnesia Haze Strain online</a>
<ahref="https://disposablecartsuk.com/product/buy-blue-gelato-41-strain-uk/" rel="dofollow">Buy Blue Gelato 41 Strain UK</a>
<ahref="https://disposablecartsuk.com/product/buy-blueberry-muffin-online-uk/" rel="dofollow">Buy Blueberry Muffin Online UK</a>
<ahref="https://disposablecartsuk.com/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-chemdawg-buy-weed-online-uk/" rel="dofollow">Buy Chemdawg – Buy Weed Online UK</a>
<ahref="https://disposablecartsuk.com/product/diamond-dust-strain/" rel="dofollow">Diamond Dust Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-gary-payton-strain/" rel="dofollow">Buy Gary Payton Strain</a>
<ahref="https://disposablecartsuk.com/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-og-kush-online/" rel="dofollow">Buy OG Kush Online</a>
<ahref="https://disposablecartsuk.com/product/permanent-marker-strain/" rel="dofollow">Permanent Marker Strain</a>
<ahref="https://disposablecartsuk.com/product/zoapscotti-strain/" rel="dofollow">Zoapscotti Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-blueberry-cake-oil-online/" rel="dofollow">Buy Blueberry Cake Oil Online</a>
<ahref="https://disposablecartsuk.com/product/co%e2%82%82-extract-oil-for-sale/" rel="dofollow">CO₂ Extract Oil For Sale</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-8-distillate-oil-online/" rel="dofollow">Buy Delta 8 Distillate Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-9-distillate-online/" rel="dofollow">Buy Delta 9 Distillate Online</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-10-distillate-oil-online/" rel="dofollow">Buy DELTA-10 Distillate Oil Online</a>
<ahref="https://disposablecartsuk.com/product/hhc-distillate/" rel="dofollow">Buy HHC Distillate Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-live-resin-oil-online/" rel="dofollow">Buy Live Resin Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-pure-raw-thc-oil-online/" rel="dofollow">Buy Pure (RAW) THC Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-purple-punch-oil-online/" rel="dofollow">Buy Purple Punch oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-rick-simpson-oil-online/" rel="dofollow">Buy Rick Simpson Oil Online</a>
<ahref="https://disposablecartsuk.com/product/thc-clear-distillate-oil-near-me/" rel="dofollow">THC Clear Distillate Oil Near Me</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-9-syrup-online/" rel="dofollow">Buy Delta 9 Syrup Online</a>
<ahref="https://disposablecartsuk.com/product/buy-thc-star-killer-oil-online/" rel="dofollow">Buy THC Star Killer Oil Online</a>
<ahref="https://disposablecartsuk.com/product/thca-distillate/" rel="dofollow">THCA Distillate</a>
<ahref="https://disposablecartsuk.com/product/buy-thcp-distillate-oil-near-me/" rel="dofollow">Buy THCP Distillate Oil Near Me</a>
<ahref="https://disposablecartsuk.com/product/whole-melt-extracts-2g-disposable/" rel="dofollow">Buy Whole Melt Extracts 2g Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/tre-house-hhc-live-resin-disposable-vape-pens-2grams/" rel="dofollow">Buy Vape Carts online uk</a>
<ahref="https://disposablecartsuk.com/product/the-wizard-of-terps-1ml-syringe/" rel="dofollow">Buy The Wizard Of Terps 1ml Syringe Online</a>
<ahref="https://disposablecartsuk.com/product/ruby-carts-disposable-vape-pen/" rel="dofollow">Buy Ruby Vape Carts online</a>
<ahref="https://disposablecartsuk.com/product/potent-disposable/" rel="dofollow">Buy Potent Disposable Vape Online UK</a>
<ahref="https://disposablecartsuk.com/product/packwoods-x-runtz/" rel="dofollow">Buy Packwoods x Runtz Vape Online UK</a>
<ahref="https://disposablecartsuk.com/product/ace-ultra-premium-disposable/" rel="dofollow">Buy Ace Ultra Premium Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/backpackboyz-carts/" rel="dofollow">Buy Backpackboyz Vape Carts Online</a>
<ahref="https://disposablecartsuk.com/product/baked-bar-2g-disposable/" rel="dofollow">Buy Baked Bar 2g Disposable Online UK</a>
<ahref="https://disposablecartsuk.com/product/burst-2g-disposable-vape/" rel="dofollow">Buy Burst 2g Disposable Vape Online</a>
<ahref="https://disposablecartsuk.com/product/cali-company-disposable-vape/" rel="dofollow">Buy Cali Company Disposable Vape Online</a>
<ahref="https://disposablecartsuk.com/product/choice-lab-2g-disposable/" rel="dofollow">Choice Lab 2g Disposable For Sale</a>
<ahref="https://disposablecartsuk.com/product/clean-carts-2g-disposable/" rel="dofollow">Buy Clean Carts 2G Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/cookies-1g-disposable/" rel="dofollow">Buy Cookies 1g Disposable Vape online</a>
<ahref="https://disposablecartsuk.com/product/family-high-range/" rel="dofollow">Buy Family High Range Vape Pens online</a>
<ahref="https://disposablecartsuk.com/product/favorites-2g-disposable/" rel="dofollow">Buy Favorites 2G Disposable Vape Online</a>
<ahref="https://disposablecartsuk.com/product/gassed-up-2g-disposable/" rel="dofollow">Buy Gassed Up 2g Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/jeeter-juice-carts/" rel="dofollow">Where can i buy vape carts in uk</a>
<ahref="https://disposablecartsuk.com/product/jungle-boys-vape/" rel="dofollow">Buy Jungle Boys Vape online</a>
<ahref="https://disposablecartsuk.com/product/seedless-disposable/" rel="dofollow">Buy disposable vape cartridges online near me</a>
<ahref="https://englandtrap.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://englandtrap.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://englandtrap.com/product/blueberry-zkittlez-strain/" rel="dofollow">Blueberry Strain</a>
<ahref="https://englandtrap.com/product/cheese-kush/" rel="dofollow">Cheese strain</a>
<ahref="https://englandtrap.com/product/gelato-strain/" rel="dofollow">Gelato Strain</a>
<ahref="https://englandtrap.com/product/gg4-strain/" rel="dofollow">GG4 Strain</a>
<ahref="https://englandtrap.com/product/godfather-og-strain/" rel="dofollow">Godfather OG Strain</a>
<ahref="https://englandtrap.com/product/stardawg-strain/" rel="dofollow">Stardawg Strain</a>
<ahref="https://englandtrap.com/product/super-lemon-cherry-strain/" rel="dofollow">Super Lemon Cherry Strain</a>
<ahref="https://englandtrap.com/product/wedding-cake-strain-2/" rel="dofollow">Wedding Cake Strain</a>
<ahref="https://englandtrap.com/product/white-widow-strain/" rel="dofollow">White Widow Strain</a>
<ahref="https://englandtrap.com/product/girl-scout-cookies/" rel="dofollow">Girl Scout Cookies</a>
<ahref="https://englandtrap.com/product/granddaddy-purple-gdp-strain/" rel="dofollow">Granddaddy Purple Strain</a>
<ahref="https://englandtrap.com/product/ice-cream-cake-strain/" rel="dofollow">Ice Cream Cake Strain</a>
<ahref="https://englandtrap.com/product/master-kush/" rel="dofollow">Master Kush</a>
<ahref="https://englandtrap.com/product/northern-lights/" rel="dofollow">Northern Lights strain</a>
<ahref="https://englandtrap.com/product/piatella-hash/" rel="dofollow">Buy Piatella Hash Online</a>
<ahref="https://englandtrap.com/product/watermelon-gelato-strain/" rel="dofollow">Watermelon Gelato Strain</a>
<ahref="https://englandtrap.com/product/dry-sift/" rel="dofollow">Buy Dry Sift Online UK</a>
<ahref="https://englandtrap.com/product/amnesia-haze/" rel="dofollow">Amnesia Haze</a>
<ahref="https://englandtrap.com/product/bruce-banner-strain/" rel="dofollow">Bruce Banner Strain</a>
<ahref="https://englandtrap.com/product/durban-poison/" rel="dofollow">Durban Poison</a>
<ahref="https://englandtrap.com/product/nepal-cream-hash/" rel="dofollow">Buy Nepal Cream Hash Online</a>
<ahref="https://englandtrap.com/product/maui-wowie-strain/" rel="dofollow">Maui Wowie Strain</a>
<ahref="https://englandtrap.com/product/cali-cream-hash/" rel="dofollow">Buy Cali Cream Hash In UK</a>
<ahref="https://englandtrap.com/product/sour-diesel-strain/" rel="dofollow">Sour Diesel Strain</a>
<ahref="https://englandtrap.com/product/super-silver-haze/" rel="dofollow">Super Silver Haze</a>
<ahref="https://englandtrap.com/product/big-chief-carts/" rel="dofollow">Big Chief Carts</a>
<ahref="https://englandtrap.com/product/cake-vape-pen/" rel="dofollow">CAKE VAPE PEN</a>
<ahref="https://englandtrap.com/product/cali-company-1g-vapes/" rel="dofollow">Cali Company 1g Vapes</a>
<ahref="https://englandtrap.com/product/dope-disposable-vape-pens/" rel="dofollow">Dope Disposable Vape Pens</a>
<ahref="https://englandtrap.com/product/amnesia-hash/" rel="dofollow">Buy Amnesia Hash Online In UK</a>
<ahref="https://englandtrap.com/product/3x-filtered-hash/" rel="dofollow">Buy 3x Filtered CBD Hash Online</a>
<ahref="https://englandtrap.com/product/fryd-vape/" rel="dofollow">FRYD VAPE</a>
<ahref="https://englandtrap.com/product/muha-meds-vape/" rel="dofollow">Muha Meds Vape</a>
<ahref="https://englandtrap.com/product/packman-vape/" rel="dofollow">PACKMAN VAPE</a>
<ahref="https://englandtrap.com/product/packwoods-vape/" rel="dofollow">PACKWOODS VAPE</a>
<ahref="https://englandtrap.com/product/cali-plates-hash/" rel="dofollow">Cali Plates Hash</a>
<ahref="https://englandtrap.com/product/fly-farm-hash/" rel="dofollow">FLY FARM HASH</a>
<ahref="https://englandtrap.com/product/16147/" rel="dofollow">Kilogrammes Farm Hash</a>
<ahref="https://englandtrap.com/product/la-mousse-hash-review/" rel="dofollow">La Mousse Hash</a>
<ahref="https://englandtrap.com/product/lemon-haze-hash/" rel="dofollow">LEMON HAZE HASH</a>
<ahref="https://englandtrap.com/product/static-room-hash/" rel="dofollow">Static Room Hash</a>
<ahref="https://englandtrap.com/product/super-pollen-hash/" rel="dofollow">Super Pollen Hash</a>
<ahref="https://englandtrap.com/product-category/cocaine/" rel="dofollow">BUY COKE ONLINE IN UK</a>
<ahref="https://englandtrap.com/product-category/cocaine/" rel="dofollow">BUY COCAINE ONLINE UK</a>
<ahref="https://englandtrap.com/product/dry-sift-hash/" rel="dofollow">Dry Sift Hash</a>
<ahref="https://englandtrap.com/product/cocaine-for-sale/" rel="dofollow">Cocaine For Sale</a>
<ahref="https://englandtrap.com/product/hashish-haschich/" rel="dofollow">HASHISH (HASCHICH)</a>
<ahref="https://englandtrap.com/product/buy-xanax-alprazolam-xr-2mg-online/" rel="dofollow">Buy Xanax (Alprazolam) XR 2mg Online</a>
<ahref="https://englandtrap.com/product/buy-xanax-alprazolam-xr-2mg-online/" rel="dofollow">Buy Xanax 2mg Online</a>
<ahref="https://englandtrap.com/product/mdmaecstasy-pills-for-sale/" rel="dofollow">MDMA(Ecstasy Pills) For Sale</a>
<ahref="https://psychedelictripgateway.com/product/native-forest-organic-mushrooms-pieces/" rel="dofollow">Native Forest Organic Mushrooms Pieces</a>
<ahref="https://psychedelictripgateway.com/product/morel-mushrooms/" rel="dofollow">Morel Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/mushroom-matsutake/" rel="dofollow">Mushroom Matsutake</a>
<ahref="https://psychedelictripgateway.com/product/dried-lobster-mushroom/" rel="dofollow">Dried Lobster Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/maitake-mushrooms/" rel="dofollow">Maitake Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/fresh-reishi-mushroom/" rel="dofollow">Fresh Reishi Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/dried-wood-ear-mushroom/" rel="dofollow">Dried Wood Ear Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/dried-black-trumpet-mushrooms/" rel="dofollow">Dried Black Trumpet Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-turkey-tail-mushroom/" rel="dofollow">Dried Turkey Tail Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-enoki-mushrooms/" rel="dofollow">Enoki Mushrooms Near Me</a>
<ahref="https://psychedelictripgateway.com/product/dried-cloud-ear-mushrooms/" rel="dofollow">Dried Cloud Ear Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-king-trumpet-mushrooms/" rel="dofollow">kings trumpet mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/white-button-mushroom-growing-kit/" rel="dofollow">White Button Mushroom growing kit</a>
<ahref="https://psychedelictripgateway.com/product/portabella-mushrooms/" rel="dofollow">Portabella Mushroom kit</a>
<ahref="https://psychedelictripgateway.com/product/mushroom-paddy-straw/" rel="dofollow">Mushroom Paddy Straw</a>
<ahref="https://psychedelictripgateway.com/product/shiitake-mushrooms-near-me/" rel="dofollow">Shiitake Mushrooms Near me</a>
<ahref="https://psychedelictripgateway.com/product/dried-chanterelle-mushrooms/" rel="dofollow">Dried Chanterelle Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/dry-porcini-mushrooms/" rel="dofollow">Dried Porcini Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/blue-oyster-mushroom-growing-kit/" rel="dofollow">Blue Oyster Mushroom Growing Kit</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-lions-mane-mushroom/" rel="dofollow">Lion’s Mane Mushroom Near Me</a>
<ahref="https://psychedelictripgateway.com/product/buy-golden-teacher-mushrooms/" rel="dofollow">Golden Teacher Mushrooms for Sale</a>
<ahref="https://psychedelictripgateway.com/product/penis-envy/" rel="dofollow">Buy Penis Envy Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-online/" rel="dofollow">Buy DMT Online</a>
<ahref="https://psychedelictripgateway.com/product/buy-1p-lsd-blotter/" rel="dofollow">Buy 1p LSD Blotter</a>
<ahref="https://psychedelictripgateway.com/product/buy-gel-tabs-lsd/" rel="dofollow">buy gel tabs lsd</a>
<ahref="https://psychedelictripgateway.com/product/buy-liquid-lsd/" rel="dofollow">Buy Liquid LSD</a>
<ahref="https://psychedelictripgateway.com/product/lsd-gel-tabs-for-sale/" rel="dofollow">Lsd Gel Tabs For Sale</a>
<ahref="https://psychedelictripgateway.com/product/buy-lsd-sheets-online/" rel="dofollow">Buy Lsd Sheets Online</a>
<ahref="https://psychedelictripgateway.com/product/4-aco-dmt-for-sale/" rel="dofollow">4 Aco DMT For Sale</a>
<ahref="https://psychedelictripgateway.com/product/n-n-dmt-for-sale/" rel="dofollow">N,N-DMT for Sale</a>
<ahref="https://psychedelictripgateway.com/product/5-meo-dmt-crystals-for-sale/" rel="dofollow">5 MEO DMT CRYSTALS FOR SALE</a>
<ahref="https://psychedelictripgateway.com/product/buy-5-bromo-dmt/" rel="dofollow">Buy 5 Bromo DMT</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-cartridges-pack-of-6/" rel="dofollow">Buy DMT Cartridges pack of 6</a>
<ahref="https://psychedelictripgateway.com/product/buy-changa-dmt/" rel="dofollow">Buy Changa DMT</a>
<ahref="https://psychedelictripgateway.com/product/pure-n-n-dmt-crystals/" rel="dofollow">Pure N,N DMT crystals(freebase)</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-powder/" rel="dofollow">Buy Dmt Powder</a>
<ahref="https://psychedelictripgateway.com/product/schwifty-labes-dmt-cartridge-5ml/" rel="dofollow">Schwifty Labes DMT (Cartridge) .5mL</a>
<ahref="https://psychedelictripgateway.com/product/deadhead-chemist-5-meo-dmt-cartridge-5ml/" rel="dofollow">Buy Deadhead Chemist 5-Meo-DMT (Cartridge) .5mL </a>
<ahref="https://psychedelictripgateway.com/product/dmt-vape-pen-double-blind/" rel="dofollow">Dmt vape pen double blind</a>
<ahref="https://psychedelictripgateway.com/product/lucid-dmt-vaporizer-nn-dmt/" rel="dofollow">Buy Lucid DMT Vaporizer (N,N- DMT) </a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-vape-cartridge-online/" rel="dofollow">Buy DMT Vape and Cartridges 1ml 400mg Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Where Can I Buy Mushroom Chocolate Bars Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy Mushroom Chocolate Bars online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Mushroom Chocolate Bars For Sale</a>
<ahref="https://psychedelictripgateway.com/product/white-yellow-technogym/" rel="dofollow">White & Yellow Technogym</a>
<ahref="https://psychedelictripgateway.com/product/orange-sprite-mdma/" rel="dofollow">Orange Sprite MDMA Pills</a>
<ahref="https://psychedelictripgateway.com/product/red-bull-258mg-mdma/" rel="dofollow">Red Bull Pills (MDMA)</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Best Store To Buy Dried Mushroom Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Order Dried Magic Mushroom Online </a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Mushroom Online USA/CANADA/UK</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Where Can I Buy Dried Mushroom Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Mushroom Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Magic Mushroom online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Dried Magic Mushroom For Sale </a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Mushroom Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy One Up Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy One Up Magic Mushroom Chocolate Bars Near me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">One Up Mushroom Chocolate Bars For Sale r</a>
<ahref="https://psychedelictripgateway.com/product/buy-lsd-online-near-me/" rel="dofollow">Buy Lsd online Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dmt-online/" rel="dofollow">DMT For Sale</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dmt-online/" rel="dofollow">Buy N,N-DMT Online</a>
<ahref="https://psychedelictripgateway.com/product/ecstasy-molly/" rel="dofollow">Ecstasy Pills Price</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-vape-pens-online/" rel="dofollow">Buy Dmt Vape Pens Online</a>
<ahref="https://psychedelictripgateway.com/product/buy-5-me0-dmt-online/" rel="dofollow">Buy 5-Me0-DMT Online</a>
<ahref="https://psychedelictripgateway.com/product/mdma-crystal/" rel="dofollow">MDMA Crystal</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Polkadot Mushroom Chocolate Bars Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy Polkadot Belgian Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Polkadot Belgian Mushroom Chocolate Bars For Sale</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Where Can I Buy Polkadot Belgian Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Where Can I Buy Polkadot Belgian Mushroom Chocolate Bars Online</a>
<ahref="https://disposablecartsuk.com/product/buy-acapulco-gold-strain-buy-premium-weed-online-in-the-uk/" rel="dofollow">Buy Acapulco Gold Strain – Buy Premium Weed Online In the UK</a>
<ahref="https://disposablecartsuk.com/product/buy-afghani-strain-online-uk/" rel="dofollow">Buy Afghani Strain Online UK</a>
<ahref="https://disposablecartsuk.com/product/buy-ak-47-strain-near-me/" rel="dofollow">Buy AK 47 Strain Near Me</a>
<ahref="https://disposablecartsuk.com/product/buy-amnesia-haze-strain-online/" rel="dofollow">Buy Amnesia Haze Strain online</a>
<ahref="https://disposablecartsuk.com/product/buy-blue-gelato-41-strain-uk/" rel="dofollow">Buy Blue Gelato 41 Strain UK</a>
<ahref="https://disposablecartsuk.com/product/buy-blueberry-muffin-online-uk/" rel="dofollow">Buy Blueberry Muffin Online UK</a>
<ahref="https://disposablecartsuk.com/product/bubblegum-popperz-strain/" rel="dofollow">Bubblegum Popperz Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-chemdawg-buy-weed-online-uk/" rel="dofollow">Buy Chemdawg – Buy Weed Online UK</a>
<ahref="https://disposablecartsuk.com/product/diamond-dust-strain/" rel="dofollow">Diamond Dust Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-gary-payton-strain/" rel="dofollow">Buy Gary Payton Strain</a>
<ahref="https://disposablecartsuk.com/product/miracle-alien-cookies-strain/" rel="dofollow">Miracle Alien Cookies Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-og-kush-online/" rel="dofollow">Buy OG Kush Online</a>
<ahref="https://disposablecartsuk.com/product/permanent-marker-strain/" rel="dofollow">Permanent Marker Strain</a>
<ahref="https://disposablecartsuk.com/product/zoapscotti-strain/" rel="dofollow">Zoapscotti Strain</a>
<ahref="https://disposablecartsuk.com/product/buy-blueberry-cake-oil-online/" rel="dofollow">Buy Blueberry Cake Oil Online</a>
<ahref="https://disposablecartsuk.com/product/co%e2%82%82-extract-oil-for-sale/" rel="dofollow">CO₂ Extract Oil For Sale</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-8-distillate-oil-online/" rel="dofollow">Buy Delta 8 Distillate Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-9-distillate-online/" rel="dofollow">Buy Delta 9 Distillate Online</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-10-distillate-oil-online/" rel="dofollow">Buy DELTA-10 Distillate Oil Online</a>
<ahref="https://disposablecartsuk.com/product/hhc-distillate/" rel="dofollow">Buy HHC Distillate Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-live-resin-oil-online/" rel="dofollow">Buy Live Resin Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-pure-raw-thc-oil-online/" rel="dofollow">Buy Pure (RAW) THC Oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-purple-punch-oil-online/" rel="dofollow">Buy Purple Punch oil Online</a>
<ahref="https://disposablecartsuk.com/product/buy-rick-simpson-oil-online/" rel="dofollow">Buy Rick Simpson Oil Online</a>
<ahref="https://disposablecartsuk.com/product/thc-clear-distillate-oil-near-me/" rel="dofollow">THC Clear Distillate Oil Near Me</a>
<ahref="https://disposablecartsuk.com/product/buy-delta-9-syrup-online/" rel="dofollow">Buy Delta 9 Syrup Online</a>
<ahref="https://disposablecartsuk.com/product/buy-thc-star-killer-oil-online/" rel="dofollow">Buy THC Star Killer Oil Online</a>
<ahref="https://disposablecartsuk.com/product/thca-distillate/" rel="dofollow">THCA Distillate</a>
<ahref="https://disposablecartsuk.com/product/buy-thcp-distillate-oil-near-me/" rel="dofollow">Buy THCP Distillate Oil Near Me</a>
<ahref="https://disposablecartsuk.com/product/whole-melt-extracts-2g-disposable/" rel="dofollow">Buy Whole Melt Extracts 2g Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/tre-house-hhc-live-resin-disposable-vape-pens-2grams/" rel="dofollow">Buy Vape Carts online uk</a>
<ahref="https://disposablecartsuk.com/product/the-wizard-of-terps-1ml-syringe/" rel="dofollow">Buy The Wizard Of Terps 1ml Syringe Online</a>
<ahref="https://disposablecartsuk.com/product/ruby-carts-disposable-vape-pen/" rel="dofollow">Buy Ruby Vape Carts online</a>
<ahref="https://disposablecartsuk.com/product/potent-disposable/" rel="dofollow">Buy Potent Disposable Vape Online UK</a>
<ahref="https://disposablecartsuk.com/product/packwoods-x-runtz/" rel="dofollow">Buy Packwoods x Runtz Vape Online UK</a>
<ahref="https://disposablecartsuk.com/product/ace-ultra-premium-disposable/" rel="dofollow">Buy Ace Ultra Premium Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/backpackboyz-carts/" rel="dofollow">Buy Backpackboyz Vape Carts Online</a>
<ahref="https://disposablecartsuk.com/product/baked-bar-2g-disposable/" rel="dofollow">Buy Baked Bar 2g Disposable Online UK</a>
<ahref="https://disposablecartsuk.com/product/burst-2g-disposable-vape/" rel="dofollow">Buy Burst 2g Disposable Vape Online</a>
<ahref="https://disposablecartsuk.com/product/cali-company-disposable-vape/" rel="dofollow">Buy Cali Company Disposable Vape Online</a>
<ahref="https://disposablecartsuk.com/product/choice-lab-2g-disposable/" rel="dofollow">Choice Lab 2g Disposable For Sale</a>
<ahref="https://disposablecartsuk.com/product/clean-carts-2g-disposable/" rel="dofollow">Buy Clean Carts 2G Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/cookies-1g-disposable/" rel="dofollow">Buy Cookies 1g Disposable Vape online</a>
<ahref="https://disposablecartsuk.com/product/family-high-range/" rel="dofollow">Buy Family High Range Vape Pens online</a>
<ahref="https://disposablecartsuk.com/product/favorites-2g-disposable/" rel="dofollow">Buy Favorites 2G Disposable Vape Online</a>
<ahref="https://disposablecartsuk.com/product/gassed-up-2g-disposable/" rel="dofollow">Buy Gassed Up 2g Disposable Online</a>
<ahref="https://disposablecartsuk.com/product/jeeter-juice-carts/" rel="dofollow">Where can i buy vape carts in uk</a>
<ahref="https://disposablecartsuk.com/product/jungle-boys-vape/" rel="dofollow">Buy Jungle Boys Vape online</a>
<ahref="https://disposablecartsuk.com/product/seedless-disposable/" rel="dofollow">Buy disposable vape cartridges online near me</a>
<ahref="https://englandtrap.com/product/apple-fritter-strain/" rel="dofollow">Apple Fritter Strain</a>
<ahref="https://englandtrap.com/product/biscotti-strain/" rel="dofollow">Biscotti Strain</a>
<ahref="https://englandtrap.com/product/blueberry-zkittlez-strain/" rel="dofollow">Blueberry Strain</a>
<ahref="https://englandtrap.com/product/cheese-kush/" rel="dofollow">Cheese strain</a>
<ahref="https://englandtrap.com/product/gelato-strain/" rel="dofollow">Gelato Strain</a>
<ahref="https://englandtrap.com/product/gg4-strain/" rel="dofollow">GG4 Strain</a>
<ahref="https://englandtrap.com/product/godfather-og-strain/" rel="dofollow">Godfather OG Strain</a>
<ahref="https://englandtrap.com/product/stardawg-strain/" rel="dofollow">Stardawg Strain</a>
<ahref="https://englandtrap.com/product/super-lemon-cherry-strain/" rel="dofollow">Super Lemon Cherry Strain</a>
<ahref="https://englandtrap.com/product/wedding-cake-strain-2/" rel="dofollow">Wedding Cake Strain</a>
<ahref="https://englandtrap.com/product/white-widow-strain/" rel="dofollow">White Widow Strain</a>
<ahref="https://englandtrap.com/product/girl-scout-cookies/" rel="dofollow">Girl Scout Cookies</a>
<ahref="https://englandtrap.com/product/granddaddy-purple-gdp-strain/" rel="dofollow">Granddaddy Purple Strain</a>
<ahref="https://englandtrap.com/product/ice-cream-cake-strain/" rel="dofollow">Ice Cream Cake Strain</a>
<ahref="https://englandtrap.com/product/master-kush/" rel="dofollow">Master Kush</a>
<ahref="https://englandtrap.com/product/northern-lights/" rel="dofollow">Northern Lights strain</a>
<ahref="https://englandtrap.com/product/piatella-hash/" rel="dofollow">Buy Piatella Hash Online</a>
<ahref="https://englandtrap.com/product/watermelon-gelato-strain/" rel="dofollow">Watermelon Gelato Strain</a>
<ahref="https://englandtrap.com/product/dry-sift/" rel="dofollow">Buy Dry Sift Online UK</a>
<ahref="https://englandtrap.com/product/amnesia-haze/" rel="dofollow">Amnesia Haze</a>
<ahref="https://englandtrap.com/product/bruce-banner-strain/" rel="dofollow">Bruce Banner Strain</a>
<ahref="https://englandtrap.com/product/durban-poison/" rel="dofollow">Durban Poison</a>
<ahref="https://englandtrap.com/product/nepal-cream-hash/" rel="dofollow">Buy Nepal Cream Hash Online</a>
<ahref="https://englandtrap.com/product/maui-wowie-strain/" rel="dofollow">Maui Wowie Strain</a>
<ahref="https://englandtrap.com/product/cali-cream-hash/" rel="dofollow">Buy Cali Cream Hash In UK</a>
<ahref="https://englandtrap.com/product/sour-diesel-strain/" rel="dofollow">Sour Diesel Strain</a>
<ahref="https://englandtrap.com/product/super-silver-haze/" rel="dofollow">Super Silver Haze</a>
<ahref="https://englandtrap.com/product/big-chief-carts/" rel="dofollow">Big Chief Carts</a>
<ahref="https://englandtrap.com/product/cake-vape-pen/" rel="dofollow">CAKE VAPE PEN</a>
<ahref="https://englandtrap.com/product/cali-company-1g-vapes/" rel="dofollow">Cali Company 1g Vapes</a>
<ahref="https://englandtrap.com/product/dope-disposable-vape-pens/" rel="dofollow">Dope Disposable Vape Pens</a>
<ahref="https://englandtrap.com/product/amnesia-hash/" rel="dofollow">Buy Amnesia Hash Online In UK</a>
<ahref="https://englandtrap.com/product/3x-filtered-hash/" rel="dofollow">Buy 3x Filtered CBD Hash Online</a>
<ahref="https://englandtrap.com/product/fryd-vape/" rel="dofollow">FRYD VAPE</a>
<ahref="https://englandtrap.com/product/muha-meds-vape/" rel="dofollow">Muha Meds Vape</a>
<ahref="https://englandtrap.com/product/packman-vape/" rel="dofollow">PACKMAN VAPE</a>
<ahref="https://englandtrap.com/product/packwoods-vape/" rel="dofollow">PACKWOODS VAPE</a>
<ahref="https://englandtrap.com/product/cali-plates-hash/" rel="dofollow">Cali Plates Hash</a>
<ahref="https://englandtrap.com/product/fly-farm-hash/" rel="dofollow">FLY FARM HASH</a>
<ahref="https://englandtrap.com/product/16147/" rel="dofollow">Kilogrammes Farm Hash</a>
<ahref="https://englandtrap.com/product/la-mousse-hash-review/" rel="dofollow">La Mousse Hash</a>
<ahref="https://englandtrap.com/product/lemon-haze-hash/" rel="dofollow">LEMON HAZE HASH</a>
<ahref="https://englandtrap.com/product/static-room-hash/" rel="dofollow">Static Room Hash</a>
<ahref="https://englandtrap.com/product/super-pollen-hash/" rel="dofollow">Super Pollen Hash</a>
<ahref="https://englandtrap.com/product-category/cocaine/" rel="dofollow">BUY COKE ONLINE IN UK</a>
<ahref="https://englandtrap.com/product-category/cocaine/" rel="dofollow">BUY COCAINE ONLINE UK</a>
<ahref="https://englandtrap.com/product/dry-sift-hash/" rel="dofollow">Dry Sift Hash</a>
<ahref="https://englandtrap.com/product/cocaine-for-sale/" rel="dofollow">Cocaine For Sale</a>
<ahref="https://englandtrap.com/product/hashish-haschich/" rel="dofollow">HASHISH (HASCHICH)</a>
<ahref="https://englandtrap.com/product/buy-xanax-alprazolam-xr-2mg-online/" rel="dofollow">Buy Xanax (Alprazolam) XR 2mg Online</a>
<ahref="https://englandtrap.com/product/buy-xanax-alprazolam-xr-2mg-online/" rel="dofollow">Buy Xanax 2mg Online</a>
<ahref="https://englandtrap.com/product/mdmaecstasy-pills-for-sale/" rel="dofollow">MDMA(Ecstasy Pills) For Sale</a>
<ahref="https://psychedelictripgateway.com/product/native-forest-organic-mushrooms-pieces/" rel="dofollow">Native Forest Organic Mushrooms Pieces</a>
<ahref="https://psychedelictripgateway.com/product/morel-mushrooms/" rel="dofollow">Morel Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/mushroom-matsutake/" rel="dofollow">Mushroom Matsutake</a>
<ahref="https://psychedelictripgateway.com/product/dried-lobster-mushroom/" rel="dofollow">Dried Lobster Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/maitake-mushrooms/" rel="dofollow">Maitake Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/fresh-reishi-mushroom/" rel="dofollow">Fresh Reishi Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/dried-wood-ear-mushroom/" rel="dofollow">Dried Wood Ear Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/dried-black-trumpet-mushrooms/" rel="dofollow">Dried Black Trumpet Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-turkey-tail-mushroom/" rel="dofollow">Dried Turkey Tail Mushroom</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-enoki-mushrooms/" rel="dofollow">Enoki Mushrooms Near Me</a>
<ahref="https://psychedelictripgateway.com/product/dried-cloud-ear-mushrooms/" rel="dofollow">Dried Cloud Ear Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-king-trumpet-mushrooms/" rel="dofollow">kings trumpet mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/white-button-mushroom-growing-kit/" rel="dofollow">White Button Mushroom growing kit</a>
<ahref="https://psychedelictripgateway.com/product/portabella-mushrooms/" rel="dofollow">Portabella Mushroom kit</a>
<ahref="https://psychedelictripgateway.com/product/mushroom-paddy-straw/" rel="dofollow">Mushroom Paddy Straw</a>
<ahref="https://psychedelictripgateway.com/product/shiitake-mushrooms-near-me/" rel="dofollow">Shiitake Mushrooms Near me</a>
<ahref="https://psychedelictripgateway.com/product/dried-chanterelle-mushrooms/" rel="dofollow">Dried Chanterelle Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/dry-porcini-mushrooms/" rel="dofollow">Dried Porcini Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/blue-oyster-mushroom-growing-kit/" rel="dofollow">Blue Oyster Mushroom Growing Kit</a>
<ahref="https://psychedelictripgateway.com/product/where-to-buy-lions-mane-mushroom/" rel="dofollow">Lion’s Mane Mushroom Near Me</a>
<ahref="https://psychedelictripgateway.com/product/buy-golden-teacher-mushrooms/" rel="dofollow">Golden Teacher Mushrooms for Sale</a>
<ahref="https://psychedelictripgateway.com/product/penis-envy/" rel="dofollow">Buy Penis Envy Mushrooms</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-online/" rel="dofollow">Buy DMT Online</a>
<ahref="https://psychedelictripgateway.com/product/buy-1p-lsd-blotter/" rel="dofollow">Buy 1p LSD Blotter</a>
<ahref="https://psychedelictripgateway.com/product/buy-gel-tabs-lsd/" rel="dofollow">buy gel tabs lsd</a>
<ahref="https://psychedelictripgateway.com/product/buy-liquid-lsd/" rel="dofollow">Buy Liquid LSD</a>
<ahref="https://psychedelictripgateway.com/product/lsd-gel-tabs-for-sale/" rel="dofollow">Lsd Gel Tabs For Sale</a>
<ahref="https://psychedelictripgateway.com/product/buy-lsd-sheets-online/" rel="dofollow">Buy Lsd Sheets Online</a>
<ahref="https://psychedelictripgateway.com/product/4-aco-dmt-for-sale/" rel="dofollow">4 Aco DMT For Sale</a>
<ahref="https://psychedelictripgateway.com/product/n-n-dmt-for-sale/" rel="dofollow">N,N-DMT for Sale</a>
<ahref="https://psychedelictripgateway.com/product/5-meo-dmt-crystals-for-sale/" rel="dofollow">5 MEO DMT CRYSTALS FOR SALE</a>
<ahref="https://psychedelictripgateway.com/product/buy-5-bromo-dmt/" rel="dofollow">Buy 5 Bromo DMT</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-cartridges-pack-of-6/" rel="dofollow">Buy DMT Cartridges pack of 6</a>
<ahref="https://psychedelictripgateway.com/product/buy-changa-dmt/" rel="dofollow">Buy Changa DMT</a>
<ahref="https://psychedelictripgateway.com/product/pure-n-n-dmt-crystals/" rel="dofollow">Pure N,N DMT crystals(freebase)</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-powder/" rel="dofollow">Buy Dmt Powder</a>
<ahref="https://psychedelictripgateway.com/product/schwifty-labes-dmt-cartridge-5ml/" rel="dofollow">Schwifty Labes DMT (Cartridge) .5mL</a>
<ahref="https://psychedelictripgateway.com/product/deadhead-chemist-5-meo-dmt-cartridge-5ml/" rel="dofollow">Buy Deadhead Chemist 5-Meo-DMT (Cartridge) .5mL </a>
<ahref="https://psychedelictripgateway.com/product/dmt-vape-pen-double-blind/" rel="dofollow">Dmt vape pen double blind</a>
<ahref="https://psychedelictripgateway.com/product/lucid-dmt-vaporizer-nn-dmt/" rel="dofollow">Buy Lucid DMT Vaporizer (N,N- DMT) </a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-vape-cartridge-online/" rel="dofollow">Buy DMT Vape and Cartridges 1ml 400mg Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Where Can I Buy Mushroom Chocolate Bars Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy Mushroom Chocolate Bars online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Mushroom Chocolate Bars For Sale</a>
<ahref="https://psychedelictripgateway.com/product/white-yellow-technogym/" rel="dofollow">White & Yellow Technogym</a>
<ahref="https://psychedelictripgateway.com/product/orange-sprite-mdma/" rel="dofollow">Orange Sprite MDMA Pills</a>
<ahref="https://psychedelictripgateway.com/product/red-bull-258mg-mdma/" rel="dofollow">Red Bull Pills (MDMA)</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Best Store To Buy Dried Mushroom Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Order Dried Magic Mushroom Online </a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Mushroom Online USA/CANADA/UK</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Where Can I Buy Dried Mushroom Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Mushroom Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Magic Mushroom online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Dried Magic Mushroom For Sale </a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dried-mushroom-online/" rel="dofollow">Buy Dried Mushroom Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy One Up Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy One Up Magic Mushroom Chocolate Bars Near me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">One Up Mushroom Chocolate Bars For Sale r</a>
<ahref="https://psychedelictripgateway.com/product/buy-lsd-online-near-me/" rel="dofollow">Buy Lsd online Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dmt-online/" rel="dofollow">DMT For Sale</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-dmt-online/" rel="dofollow">Buy N,N-DMT Online</a>
<ahref="https://psychedelictripgateway.com/product/ecstasy-molly/" rel="dofollow">Ecstasy Pills Price</a>
<ahref="https://psychedelictripgateway.com/product/buy-dmt-vape-pens-online/" rel="dofollow">Buy Dmt Vape Pens Online</a>
<ahref="https://psychedelictripgateway.com/product/buy-5-me0-dmt-online/" rel="dofollow">Buy 5-Me0-DMT Online</a>
<ahref="https://psychedelictripgateway.com/product/mdma-crystal/" rel="dofollow">MDMA Crystal</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Polkadot Mushroom Chocolate Bars Near Me</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Buy Polkadot Belgian Mushroom Chocolate Bars Online</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Polkadot Belgian Mushroom Chocolate Bars For Sale</a>
<ahref="https://psychedelictripgateway.com/product-category/buy-mushroom-chocolate-bars-online/" rel="dofollow">Where Can I Buy Polkadot Belgian Mushroom Chocolate Bars Online</a>
If you are going for finest contents like myself, just pay a quick visit this site everyday for the reason that it offers quality contents, thanks
I'm gone to tell my little brother, that he should also go to see this website on regular basis to get updated from most recent information.
You actually expressed it superbly.
You definitely made the point.
You have made the point!
If some one desires expert view regarding running a blog after that i propose him/her to visit this weblog, Keep up the good work.
You have made the point!
You’re a master of the written word!
Tips very well considered!
Hi are using Wordpress for your blog platform? I'm new to the blog world but I'm trying to get started and set up my own. Do you need any html coding expertise to make your own blog? Any help would be really appreciated!
You actually expressed it superbly.
Nicely put, Cheers.
Good post. I learn something totally new and challenging on blogs I stumbleupon every day. It's always helpful to read content from other authors and practice something from their web sites.
Very nice post. I just stumbled upon your weblog and wanted to say that I have truly enjoyed browsing your weblog posts. After all I'll be subscribing in your rss feed and I'm hoping you write once more very soon!
Greetings! Quick question that's totally off topic. Do you know how to make your site mobile friendly? My weblog looks weird when viewing from my iphone 4. I'm trying to find a template or plugin that might be able to resolve this problem. If you have any recommendations, please share. Thank you!
Hey there just wanted to give you a quick heads up. The text in your content seem to be running off the screen in Safari. I'm not sure if this is a formatting issue or something to do with web browser compatibility but I figured I'd post to let you know. The design and style look great though! Hope you get the issue solved soon. Many thanks
I've been browsing online more than 2 hours today, yet I never found any interesting article like yours. It's pretty worth enough for me. In my opinion, if all site owners and bloggers made good content as you did, the web will be a lot more useful than ever before.
I've read a few good stuff here. Definitely worth bookmarking for revisiting. I wonder how so much effort you place to create any such great informative web site.
Hello, after reading this remarkable article i am also happy to share my familiarity here with mates.
I like what you guys tend to be up too. Such clever work and reporting! Keep up the fantastic works guys I've included you guys to my personal blogroll.
My developer is trying to convince me to move to .net from PHP. I have always disliked the idea because of the costs. But he's tryiong none the less. I've been using Movable-type on a variety of websites for about a year and am nervous about switching to another platform. I have heard very good things about blogengine.net. Is there a way I can transfer all my wordpress posts into it? Any kind of help would be really appreciated!
Link exchange is nothing else but it is only placing the other person's webpage link on your page at proper place and other person will also do same in favor of you.
Very quickly this web site will be famous among all blog visitors, due to it's pleasant articles
You have made the point!
Good stuff. Cheers!
Very good
You definitely made the point.
Very nice write-up. I absolutely love this website. Thanks!
Hey! This is kind of off topic but I need some advice from an established blog. Is it tough to set up your own blog? I'm not very techincal but I can figure things out pretty fast. I'm thinking about making my own but I'm not sure where to begin. Do you have any tips or suggestions? Cheers
Oh my goodness! Impressive article dude! Thanks, However I am experiencing issues with your RSS. I don't understand why I cannot subscribe to it. Is there anybody getting the same RSS issues? Anyone that knows the answer can you kindly respond? Thanx!!
I just could not go away your website before suggesting that I extremely enjoyed the usual info a person supply on your guests? Is gonna be again frequently to check up on new posts
This is my first time go to see at here and i am actually pleassant to read all at single place.
I think this is among the most vital info for me. And i am glad reading your article. But wanna remark on some general things, The web site style is great, the articles is really great : D. Good job, cheers
The other day, while I was at work, my sister stole my iphone and tested to see if it can survive a forty foot drop, just so she can be a youtube sensation. My apple ipad is now broken and she has 83 views. I know this is totally off topic but I had to share it with someone!
I will right away grasp your rss as I can not to find your e-mail subscription link or newsletter service. Do you’ve any? Kindly let me recognise so that I could subscribe. Thanks.
I am truly pleased to glance at this website posts which carries plenty of helpful facts, thanks for providing such information.
I don't even know how I ended up here, but I thought this post was great. I don't know who you are but definitely you are going to a famous blogger if you are not already ;) Cheers!
Hmm is anyone else encountering problems with the images on this blog loading? I'm trying to determine if its a problem on my end or if it's the blog. Any feed-back would be greatly appreciated.
Do you have a spam problem on this site; I also am a blogger, and I was curious about your situation; we have developed some nice procedures and we are looking to exchange solutions with others, why not shoot me an e-mail if interested.
Good replies in return of this query with real arguments and explaining the whole thing on the topic of that.
Please let me know if you're looking for a article author for your weblog. You have some really great posts and I believe I would be a good asset. If you ever want to take some of the load off, I'd love to write some material for your blog in exchange for a link back to mine. Please shoot me an email if interested. Regards!
Your style is so unique in comparison to other folks I have read stuff from. Thank you for posting when you have the opportunity, Guess I will just book mark this web site.
Hello there! Do you know if they make any plugins to assist with SEO? I'm trying to get my blog to rank for some targeted keywords but I'm not seeing very good success. If you know of any please share. Cheers!
I do agree with all the ideas you have introduced to your post. They are very convincing and will certainly work. Still, the posts are too brief for novices. May just you please lengthen them a bit from subsequent time? Thanks for the post.
You have made some good points there. I looked on the web to learn more about the issue and found most people will go along with your views on this web site.
I'll right away grasp your rss feed as I can't to find your e-mail subscription link or newsletter service. Do you've any? Kindly permit me realize in order that I may just subscribe. Thanks.
You’re a master of the written word!
Amazing plenty of good information.
Tips very well considered!
Superb postings. With thanks.
Very good
Amazing plenty of good information.
Hello there, I found your site by way of Google while searching for a comparable matter, your website got here up, it appears to be like great. I've bookmarked it in my google bookmarks.
Hello there, simply changed into alert to your weblog through Google, and located that it is really informative. I'm going to be careful for brussels. I will appreciate should you continue this in future. A lot of people can be benefited out of your writing. Cheers!
It's amazing to pay a quick visit this site and reading the views of all colleagues about this paragraph, while I am also keen of getting know-how.
Hello colleagues, pleasant post and pleasant urging commented at this place, I am in fact enjoying by these.
You actually expressed it superbly.
Nicely put, Cheers.
You definitely made the point.
You have made the point!
Nicely put, Cheers.
You actually expressed it superbly.
Nicely put, Cheers.
Nicely put, Cheers.
magnificent issues altogether, you just received a emblem new reader. What could you recommend about your post that you simply made some days in the past? Any certain?
Amazing plenty of good information.
Good stuff. Cheers!
You actually expressed it superbly.
Hello, just wanted to mention, I loved this article. It was inspiring. Keep on posting!
Superb postings. With thanks.
Superb postings. With thanks.
Amazing plenty of good information.
Hey There. I found your blog using msn. This is a very well written article. I'll be sure to bookmark it and return to read more of your useful information. Thanks for the post. I will certainly return.
What's up mates, how is everything, and what you desire to say on the topic of this paragraph, in my view its really remarkable designed for me.
You actually expressed it superbly.
Good stuff. Cheers!
Nicely put, Cheers.
You said it perfectly.
You’re a master of the written word!
Very good
Superb postings. With thanks.
Quality content is the secret to be a focus for the people to go to see the site, that's what this website is providing.
Nicely put, Cheers.
Good stuff. Cheers!
You actually expressed it superbly.
Hey! I know this is kinda off topic but I'd figured I'd ask. Would you be interested in trading links or maybe guest writing a blog post or vice-versa? My website discusses a lot of the same subjects as yours and I feel we could greatly benefit from each other. If you happen to be interested feel free to shoot me an email. I look forward to hearing from you! Superb blog by the way!
It's difficult to find well-informed people in this particular topic, however, you seem like you know what you're talking about! Thanks
Leave a Comment